Design, Synthesis, and Biological Evaluation of Boron-Containing Macrocyclic Polyamines and Their Zinc(II) Complexes for Boron Neutron Capture Therapy
- PMID: 34077212
- PMCID: PMC8279495
- DOI: 10.1021/acs.jmedchem.1c00445
Design, Synthesis, and Biological Evaluation of Boron-Containing Macrocyclic Polyamines and Their Zinc(II) Complexes for Boron Neutron Capture Therapy
Abstract
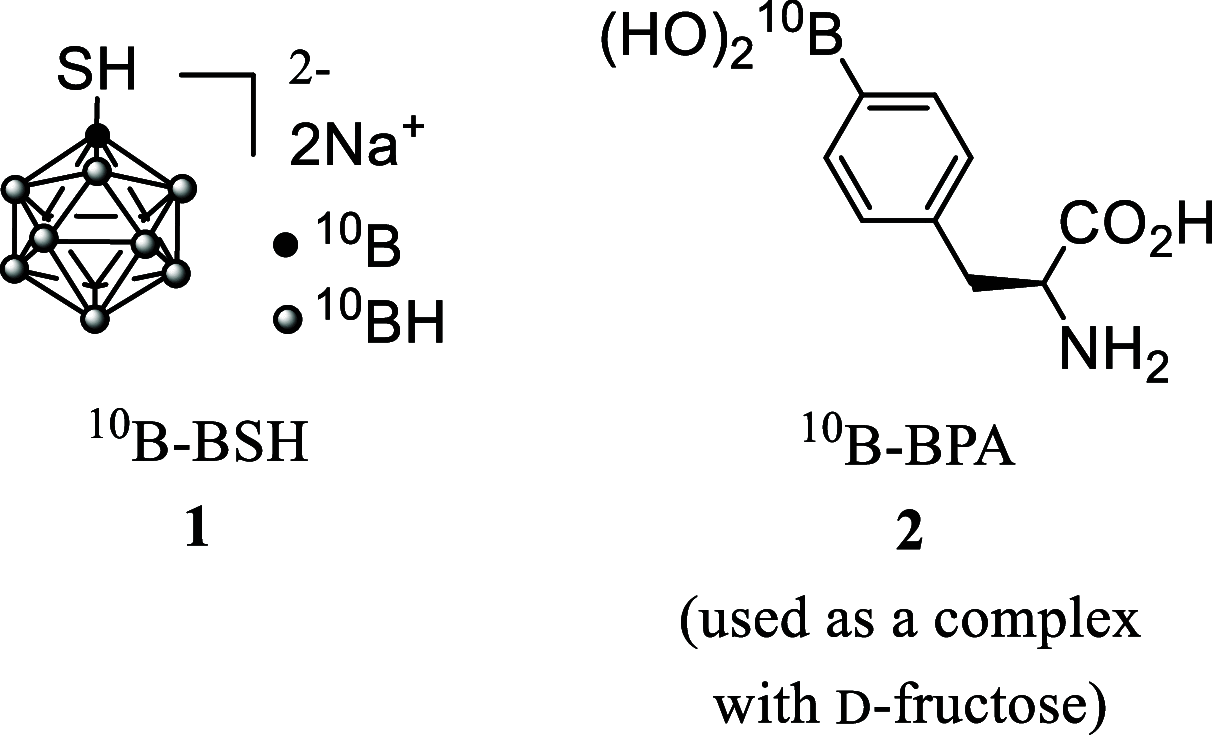
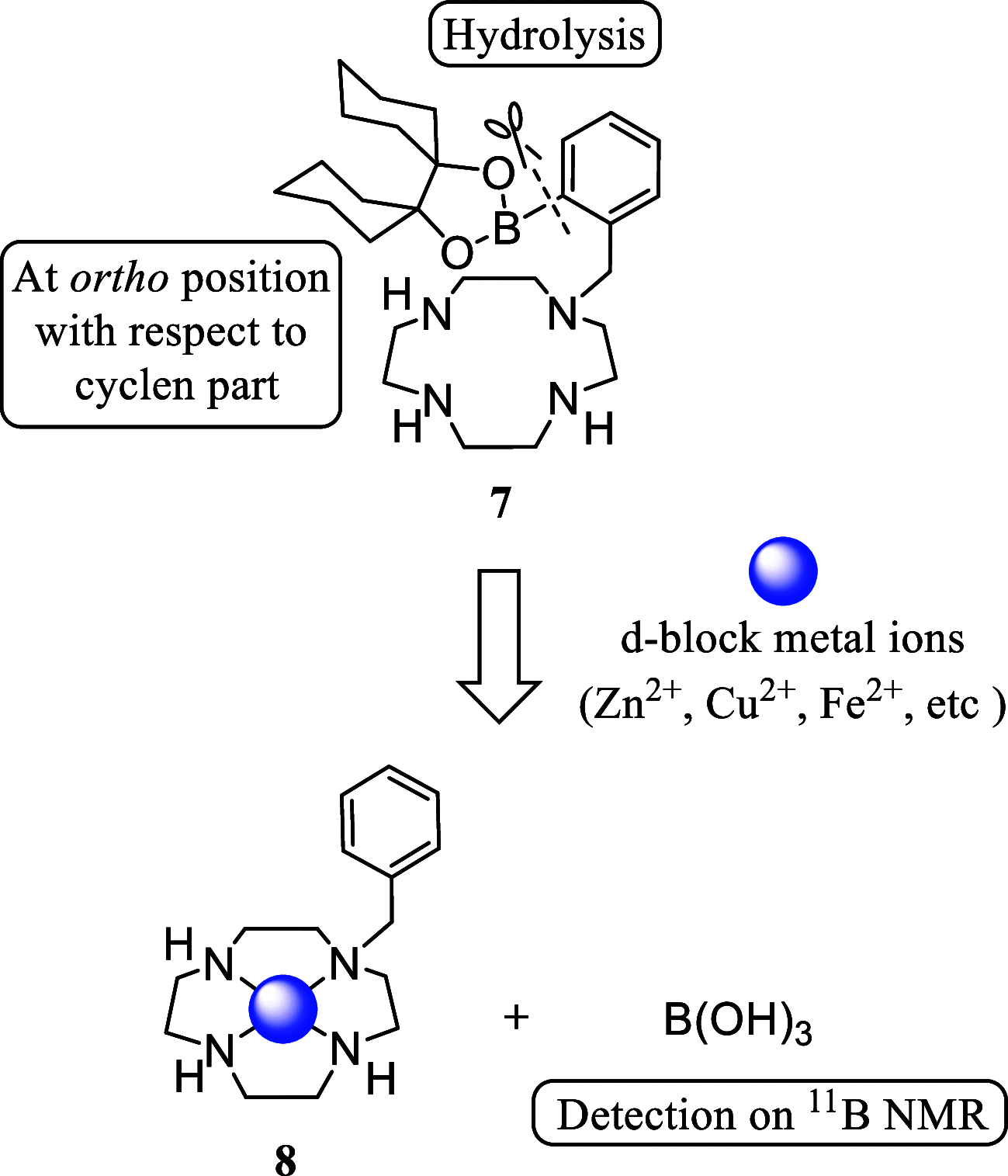
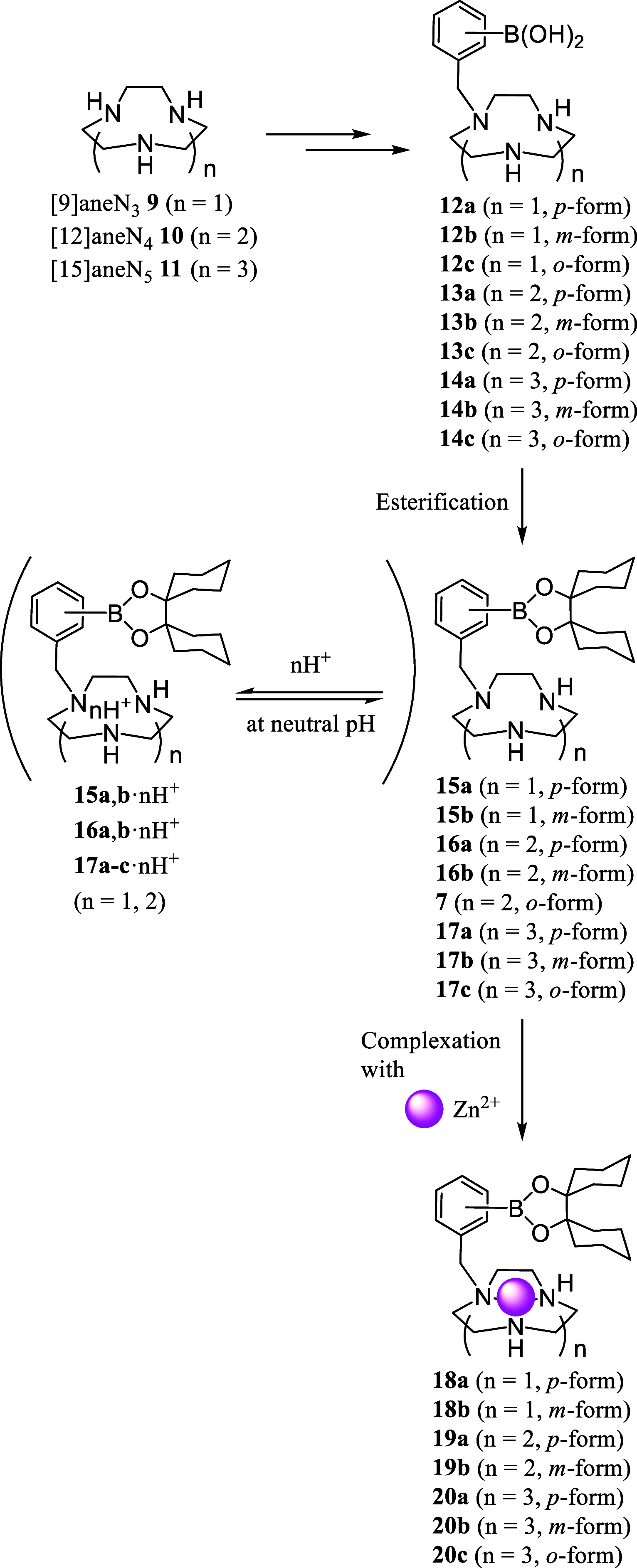
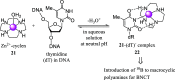
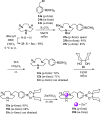
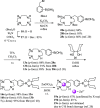
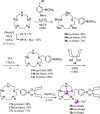
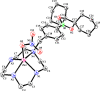
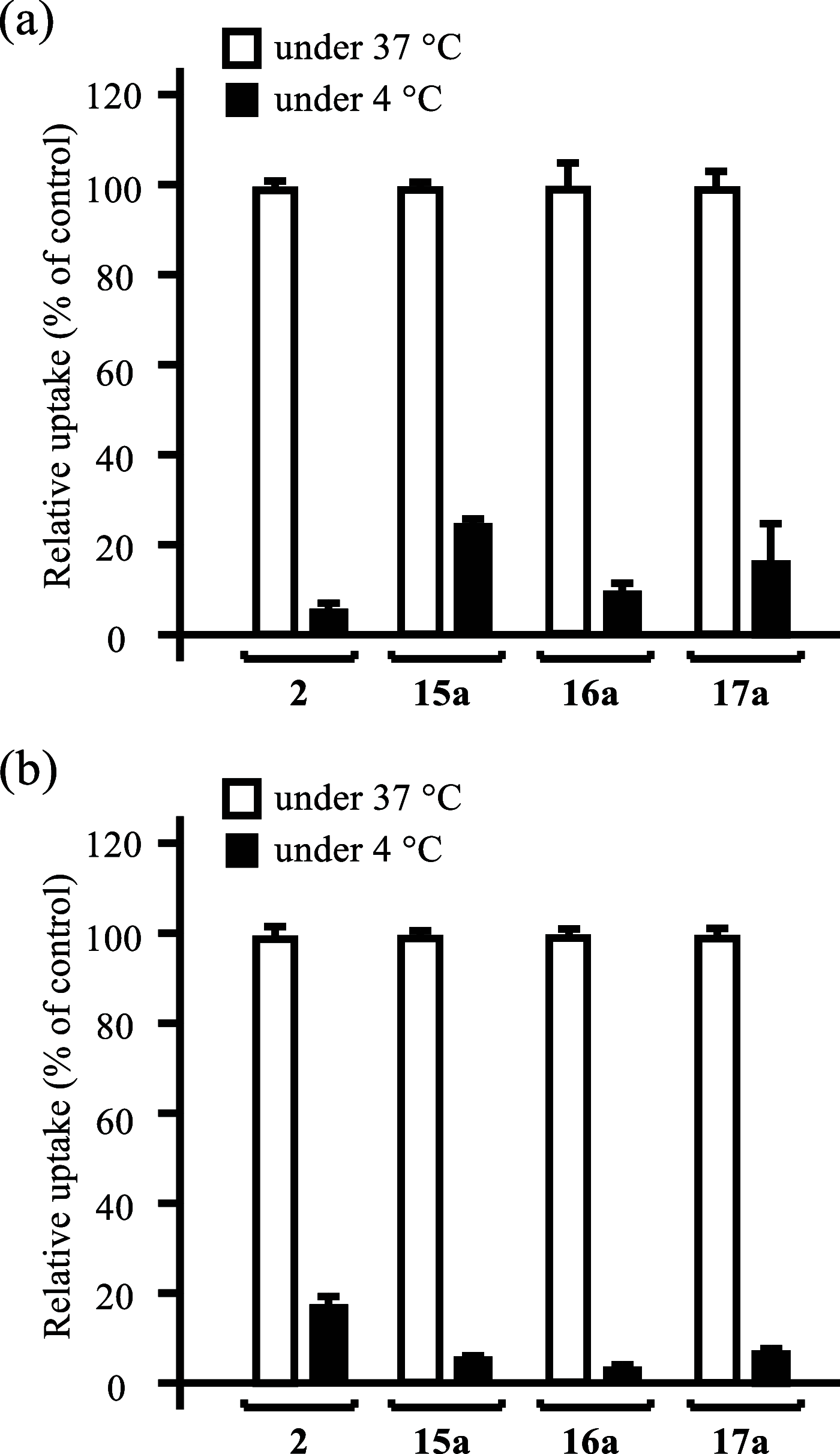
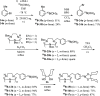
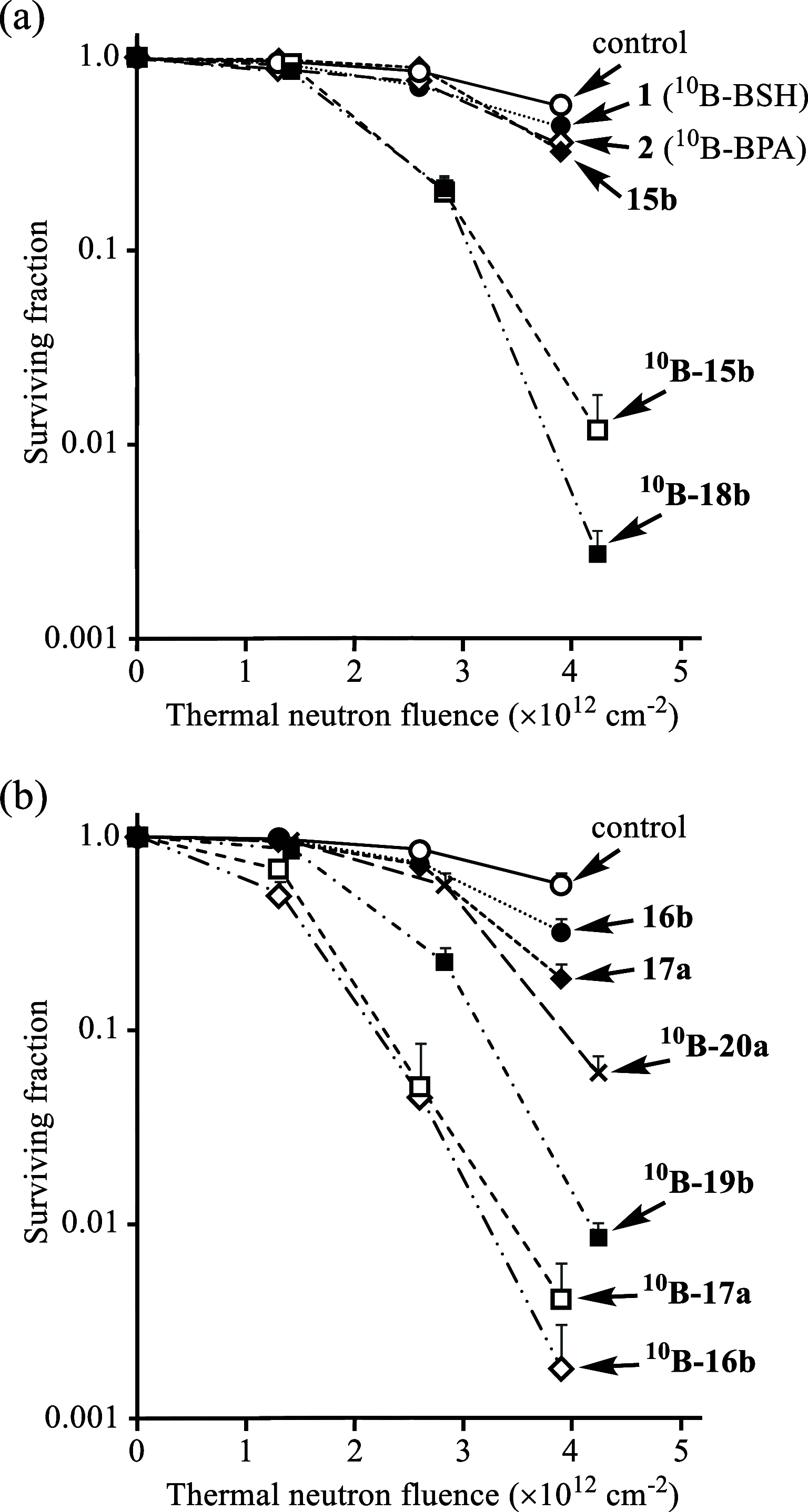
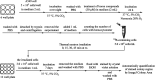
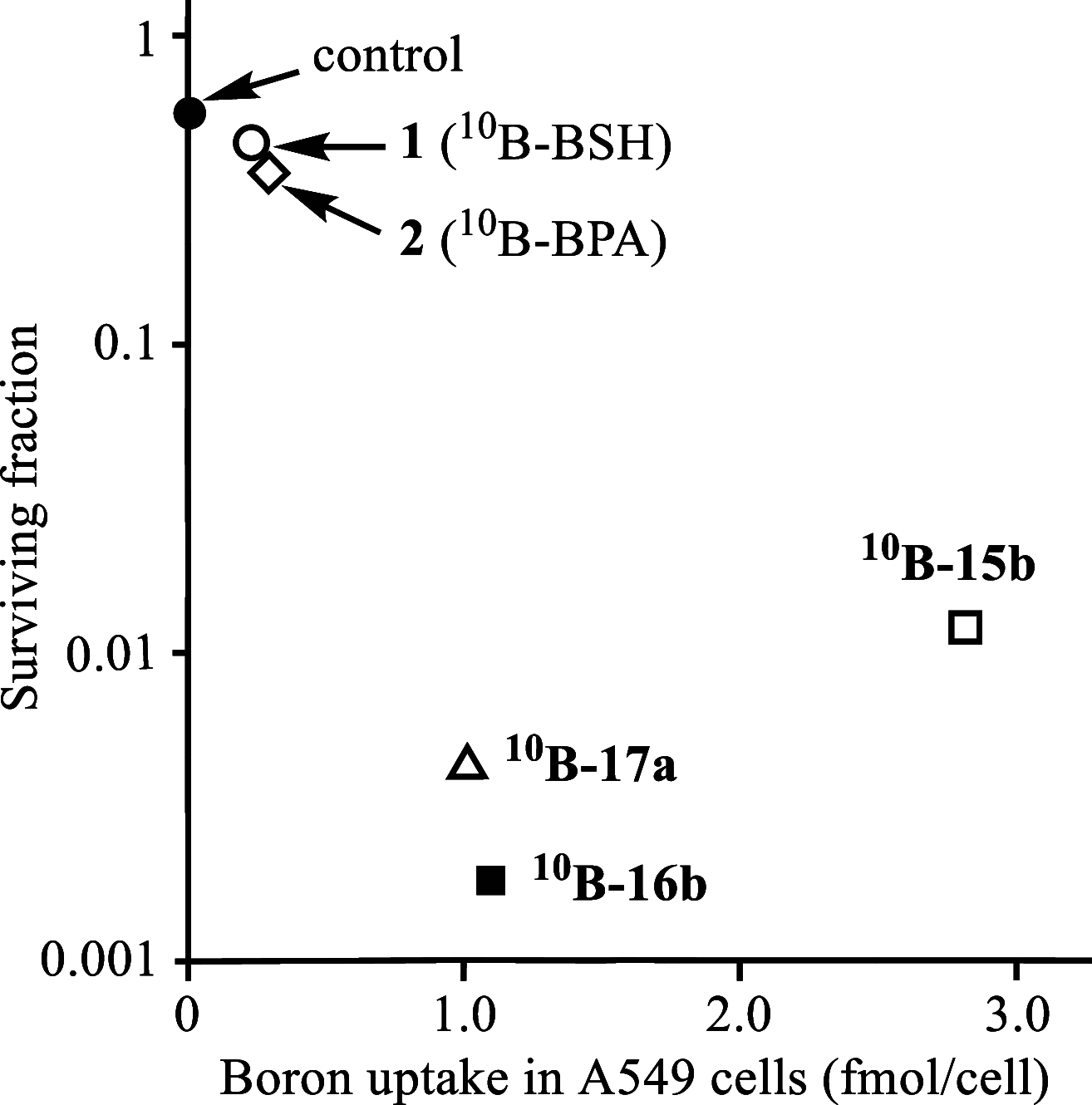
Boron neutron capture therapy (BNCT) is a binary therapeutic method for cancer treatment based on the use of a combination of a cancer-specific drug containing boron-10 (10B) and thermal neutron irradiation. For successful BNCT, 10B-containing molecules need to accumulate specifically in cancer cells, because destructive effect of the generated heavy particles is limited basically to boron-containing cells. Herein, we report on the design and synthesis of boron compounds that are functionalized with 9-, 12-, and 15-membered macrocyclic polyamines and their Zn2+ complexes. Their cytotoxicity, intracellular uptake activity into cancer cells and normal cells, and BNCT effect are also reported. The experimental data suggest that mono- and/or diprotonated forms of metal-free [12]aneN4- and [15]aneN5-type ligands are uptaken into cancer cells, and their complexes with intracellular metals such as Zn2+ would induce cell death upon thermal neutron irradiation, possibly via interactions with DNA.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Water-soluble carboranyl-phthalocyanines for BNCT. Synthesis, characterization, and in vitro tests of the Zn(II)-nido-carboranyl-hexylthiophthalocyanine.Dalton Trans. 2015 Jun 28;44(24):11021-8. doi: 10.1039/c5dt00394f. Epub 2015 May 21. Dalton Trans. 2015. PMID: 25995094
-
Synthesis, characterization and biological evaluation of carboranylmethylbenzo[b]acridones as novel agents for boron neutron capture therapy.Org Biomol Chem. 2014 Jul 28;12(28):5201-11. doi: 10.1039/c4ob00644e. Org Biomol Chem. 2014. PMID: 24915168
-
Tumor Cell-Specific 2'-Fluoro RNA Aptamer Conjugated with Closo-Dodecaborate as A Potential Agent for Boron Neutron Capture Therapy.Int J Mol Sci. 2021 Jul 7;22(14):7326. doi: 10.3390/ijms22147326. Int J Mol Sci. 2021. PMID: 34298946 Free PMC article.
-
The synthesis and use of boronated amino acids for boron neutron capture therapy.Anticancer Agents Med Chem. 2006 Mar;6(2):111-25. doi: 10.2174/187152006776119144. Anticancer Agents Med Chem. 2006. PMID: 16529535 Review.
-
Boron Agent Delivery Platforms Based on Natural Products for Boron Neutron Capture Therapy.ChemMedChem. 2024 Nov 4;19(21):e202400323. doi: 10.1002/cmdc.202400323. Epub 2024 Oct 15. ChemMedChem. 2024. PMID: 38830821 Review.
Cited by
-
Investigation of the Impact of L-Phenylalanine and L-Tyrosine Pre-Treatment on the Uptake of 4-Borono-L-Phenylalanine in Cancerous and Normal Cells Using an Analytical Approach Based on SC-ICP-MS.Molecules. 2023 Sep 10;28(18):6552. doi: 10.3390/molecules28186552. Molecules. 2023. PMID: 37764328 Free PMC article.
-
Novel Drug Delivery Particles Can Provide Dual Effects on Cancer "Theranostics" in Boron Neutron Capture Therapy.Cells. 2025 Jan 6;14(1):60. doi: 10.3390/cells14010060. Cells. 2025. PMID: 39791761 Free PMC article.
-
Advances in the synthesis and applications of macrocyclic polyamines.R Soc Open Sci. 2024 Jun 19;11(6):231979. doi: 10.1098/rsos.231979. eCollection 2024 Jun. R Soc Open Sci. 2024. PMID: 39092147 Free PMC article. Review.
References
-
- Barth R. F.; Soloway A. H.; Fairchild R. G. Boron Neutron Capture Therapy of Cancer. Cancer Res. 1990, 50, 1061–1070. - PubMed
- Salt C.; Lennox A. J.; Takagaki M.; Maguire J. A.; Hosmane N. S. Boron and Gadolinium Neutron Capture Therapy. Russ. Chem. Bull. 2004, 53, 1871–1888. 10.1007/s11172-005-0045-6. - DOI
- Ali F.; S Hosmane N.; Zhu Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828.10.3390/molecules25040828. - DOI - PMC - PubMed
-
- Hawthorne M. F. The Role of Chemistry in the Development of Boron Neutron Capture Therapy of Cancer. Angew. Chem., Int. Ed. Engl. 1993, 32, 950–984. 10.1002/anie.199309501. - DOI
- Morin C. The Chemistry of Boron Analogues of Biomolecules. Tetrahedron 1994, 50, 12521–12569. 10.1016/s0040-4020(01)89389-3. - DOI
- Soloway A. H.; Tjarks W.; Barnum B. A.; Rong F.-G.; Barth R. F.; Codogni I. M.; Wilson J. G. The Chemistry of Neutron Capture Therapy. Chem. Rev. 1998, 98, 1515–1562. 10.1021/cr941195u. - DOI - PubMed
- Barth R. F.; Coderre J. A.; Vicente M. G. H.; Blue T. E. Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects. Clin. Cancer Res. 2005, 11, 3987–4002. 10.1158/1078-0432.ccr-05-0035. - DOI - PubMed
- Luderer M. J.; de la Puente P.; Azab A. K. Advancements in Tumor Targeting Strategies for Boron Neutron Capture Therapy. Pharm. Res. 2015, 32, 2824–2836. 10.1007/s11095-015-1718-y. - DOI - PubMed
- Barth R. F.; Mi P.; Yang W. Boron Delivery Agents for Boron Neutron Capture Therapy of Cancer. Canc. Commun. 2018, 38, 35.10.1186/s40880-018-0299-7. - DOI - PMC - PubMed
- Cerecetto H.; Couto M.. Medicinal Chemistry of Boron-Bearing Compounds for BNCT-Glioma Treatment: Current Challenges and Perspectives. In Glioma—Contemporary Diagnostic and Therapeutic Approaches; Omerhodžić I., Arnautović K., Eds.; IntechOpen: U.K., 2018.
-
- Snyder H. R.; Reedy A. J.; Lennarz W. J. Synthesis of Aromatic Boronic Acids. Aldehydo Boronic Acids and a Boronic Acid Analog of Tyrosine. J. Am. Chem. Soc. 1958, 80, 835–838. 10.1021/ja01537a021. - DOI
- Shull B. K.; Spielvogel D. E.; Head G.; Gopalaswamy R.; Sankar S.; Devito K. Studies on the Structure of the Complex of the Boron Neutron Capture Therapy Drug, L-p-Boronophenylalanine, with Fructose and Related Carbohydrates: Chemical and C NMR Evidence for the β-D-Fructofuranose 2,3,6-(p-Phenylalanylorthoboronate) Structure. J. Pharm. Sci. 2000, 89, 215–222. 10.1002/(sici)1520-6017(200002)89:2<215::aid-jps8>3.3.co;2-g. - DOI - PubMed
- Wongthai P.; Hagiwara K.; Miyoshi Y.; Wiriyasermkul P.; Wei L.; Ohgaki R.; Kato I.; Hamase K.; Nagamori S.; Kanai Y. Boronophenylalanine, a Boron Delivery agent for Boron Neutron Capture Therapy, is Transported by ATBo,+, LAT1 and LAT2. Cancer Sci. 2015, 106, 279–286. 10.1111/cas.12602. - DOI - PMC - PubMed
- Watanabe T.; Hattori Y.; Ohta Y.; Ishimura M.; Nakagawa Y.; Sanada Y.; Tanaka H.; Fukutani S.; Masunaga S.; Hiraoka M.; Ono K.; Suzuki M.; Kirihata M. Comparison of the Pharmacokinetics Between L-BPA and L-FBPA Using the Same Administration Dose and Protocol: a Validation Study for the Theranostic Approach Using [18F]-L-BPA Positron Emission Tomography in Boron Neutron Capture Therapy. BMC Canc. 2016, 16, 859.10.1186/s12885-016-2913-x. - DOI - PMC - PubMed
-
- Barth R. F.; Vicente M. G. H.; Harling O. K.; Kinger W. S. III; Riley K. J.; Binns P. J.; Wagner F. M.; Suzuki M.; Aihara T.; Kato I.; Kawabata S. Current Status of Boron Neutron Capture Therapy of High Grade Gliomas and Recurrent Head and Neck Cancer. Radiat. Oncol. 2012, 7, 146.10.1186/1748-717x-7-146. - DOI - PMC - PubMed
- Suzuki M.; Kato I.; Aihara T.; Hiratsuka J.; Yoshimura K.; Niimi M.; Kimura Y.; Ariyoshi Y.; Haginomori S.-i.; Sakurai Y.; Kinashi Y.; Masunaga S.-i.; Fukushima M.; Ono K.; Maruhashi A. Boron Neutron Capture Therapy Outcomes for Advanced or Recurrent Head and Neck Cancer. J. Radiat. Res. 2014, 55, 146–153. 10.1093/jrr/rrt098. - DOI - PMC - PubMed
- Barth R. F.; Zhang Z.; Liu T. A Realistic Appraisal of Boron Neutron Capture Therapy as a Cancer Treatment Nodality. Canc. Commun. 2018, 38, 36.10.1186/s40880-018-0280-5. - DOI - PMC - PubMed
- Suzuki M. Boron Neutron Capture Therapy (BNCT): a Unique Role in Radiotherapy with a View to Entering the Accelerator-based BNCT era. Int. J. Clin. Oncol. 2020, 25, 43–50. 10.1007/s10147-019-01480-4. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

