Randomized Phase II Study of PET Response-Adapted Combined Modality Therapy for Esophageal Cancer: Mature Results of the CALGB 80803 (Alliance) Trial
- PMID: 34077237
- PMCID: PMC8407649
- DOI: 10.1200/JCO.20.03611
Randomized Phase II Study of PET Response-Adapted Combined Modality Therapy for Esophageal Cancer: Mature Results of the CALGB 80803 (Alliance) Trial
Abstract
Purpose: To evaluate the use of early assessment of chemotherapy responsiveness by positron emission tomography (PET) imaging to tailor therapy in patients with esophageal and esophagogastric junction adenocarcinoma.
Methods: After baseline PET, patients were randomly assigned to an induction chemotherapy regimen: modified oxaliplatin, leucovorin, and fluorouracil (FOLFOX) or carboplatin-paclitaxel (CP). Repeat PET was performed after induction; change in maximum standardized uptake value (SUV) from baseline was assessed. PET nonresponders (< 35% decrease in SUV) crossed over to the alternative chemotherapy during chemoradiation (50.4 Gy/28 fractions). PET responders (≥ 35% decrease in SUV) continued on the same chemotherapy during chemoradiation. Patients underwent surgery at 6 weeks postchemoradiation. Primary end point was pathologic complete response (pCR) rate in nonresponders after switching chemotherapy.
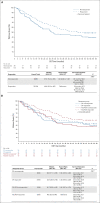
Results: Two hundred forty-one eligible patients received Protocol treatment, of whom 225 had an evaluable repeat PET. The pCR rates for PET nonresponders after induction FOLFOX who crossed over to CP (n = 39) or after induction CP who changed to FOLFOX (n = 50) was 18.0% (95% CI, 7.5 to 33.5) and 20% (95% CI, 10 to 33.7), respectively. The pCR rate in responders who received induction FOLFOX was 40.3% (95% CI, 28.9 to 52.5) and 14.1% (95% CI, 6.6 to 25.0) in responders to CP. With a median follow-up of 5.2 years, median overall survival was 48.8 months (95% CI, 33.2 months to not estimable) for PET responders and 27.4 months (95% CI, 19.4 months to not estimable) for nonresponders. For induction FOLFOX patients who were PET responders, median survival was not reached.
Conclusion: Early response assessment using PET imaging as a biomarker to individualize therapy for patients with esophageal and esophagogastric junction adenocarcinoma was effective, improving pCR rates in PET nonresponders. PET responders to induction FOLFOX who continued on FOLFOX during chemoradiation achieved a promising 5-year overall survival of 53%.
Trial registration: ClinicalTrials.gov NCT01333033.
Conflict of interest statement
Figures

Comment in
-
[Randomized phase II study of PET response-adapted combined multimodal therapy for esophageal cancer: results of the CALGB 80803 (Alliance) trial].Strahlenther Onkol. 2022 Mar;198(3):322-324. doi: 10.1007/s00066-021-01898-8. Epub 2022 Jan 11. Strahlenther Onkol. 2022. PMID: 35015087 Free PMC article. German. No abstract available.
-
CROSSing into New Therapies for Esophageal Cancer.Int J Radiat Oncol Biol Phys. 2022 May 1;113(1):5-10. doi: 10.1016/j.ijrobp.2021.12.177. Int J Radiat Oncol Biol Phys. 2022. PMID: 35427559 No abstract available.
References
-
- Arnold M, Laversanne M, Brown LM.Predicting the future burden of esophageal cancer by histological subtype: International trends in incidence up to 2030 Am J Gastroenterol 1121247–12552017 - PubMed
-
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer N Engl J Med 3662074–20842012 - PubMed
-
- Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial Lancet Oncol 161090–10982015 - PubMed
-
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: A Japan Clinical Oncology Group Study—JCOG9204 J Clin Oncol 214592–45962003 - PubMed
-
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907) Ann Surg Oncol 1968–742012 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

