Clinicopathologic Features and Response to Therapy of NRG1 Fusion-Driven Lung Cancers: The eNRGy1 Global Multicenter Registry
- PMID: 34077268
- PMCID: PMC8407651
- DOI: 10.1200/JCO.20.03307
Clinicopathologic Features and Response to Therapy of NRG1 Fusion-Driven Lung Cancers: The eNRGy1 Global Multicenter Registry
Abstract
Purpose: Although NRG1 fusions are oncogenic drivers across multiple tumor types including lung cancers, these are difficult to study because of their rarity. The global eNRGy1 registry was thus established to characterize NRG1 fusion-positive lung cancers in the largest and most diverse series to date.
Methods: From June 2018 to February 2020, a consortium of 22 centers from nine countries in Europe, Asia, and the United States contributed data from patients with pathologically confirmed NRG1 fusion-positive lung cancers. Profiling included DNA-based and/or RNA-based next-generation sequencing and fluorescence in situ hybridization. Anonymized clinical, pathologic, molecular, and response (RECIST v1.1) data were centrally curated and analyzed.
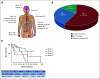
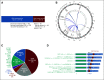
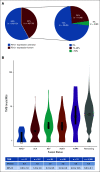
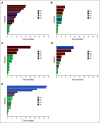
Results: Although the typified never smoking (57%), mucinous adenocarcinoma (57%), and nonmetastatic (71%) phenotype predominated in 110 patients with NRG1 fusion-positive lung cancer, further diversity, including in smoking history (43%) and histology (43% nonmucinous and 6% nonadenocarcinoma), was elucidated. RNA-based testing identified most fusions (74%). Molecularly, six (of 18) novel 5' partners, 20 unique epidermal growth factor domain-inclusive chimeric events, and heterogeneous 5'/3' breakpoints were found. Platinum-doublet and taxane-based (post-platinum-doublet) chemotherapy achieved low objective response rates (ORRs 13% and 14%, respectively) and modest progression-free survival medians (PFS 5.8 and 4.0 months, respectively). Consistent with a low programmed death ligand-1 expressing (28%) and low tumor mutational burden (median: 0.9 mutations/megabase) immunophenotype, the activity of chemoimmunotherapy and single-agent immunotherapy was poor (ORR 0%/PFS 3.3 months and ORR 20%/PFS 3.6 months, respectively). Afatinib achieved an ORR of 25%, not contingent on fusion type, and a 2.8-month median PFS.
Conclusion: NRG1 fusion-positive lung cancers were molecularly, pathologically, and clinically more heterogeneous than previously recognized. The activity of cytotoxic, immune, and targeted therapies was disappointing. Further research examining NRG1-rearranged tumor biology is needed to develop new therapeutic strategies.
Conflict of interest statement
Figures
Similar articles
-
Therapeutic Potential of Afatinib in NRG1 Fusion-Driven Solid Tumors: A Case Series.Oncologist. 2021 Jan;26(1):7-16. doi: 10.1634/theoncologist.2020-0379. Epub 2020 Sep 23. Oncologist. 2021. PMID: 32852072 Free PMC article.
-
Identification of tumors with NRG1 rearrangement, including a novel putative pathogenic UNC5D-NRG1 gene fusion in prostate cancer by data-drilling a de-identified tumor database.Genes Chromosomes Cancer. 2021 Jul;60(7):474-481. doi: 10.1002/gcc.22942. Epub 2021 Feb 24. Genes Chromosomes Cancer. 2021. PMID: 33583086
-
Successful targeting of the NRG1 pathway indicates novel treatment strategy for metastatic cancer.Ann Oncol. 2017 Dec 1;28(12):3092-3097. doi: 10.1093/annonc/mdx523. Ann Oncol. 2017. PMID: 28950338
-
NRG1 fusion-driven tumors: biology, detection, and the therapeutic role of afatinib and other ErbB-targeting agents.Ann Oncol. 2020 Dec;31(12):1693-1703. doi: 10.1016/j.annonc.2020.08.2335. Epub 2020 Sep 9. Ann Oncol. 2020. PMID: 32916265 Free PMC article. Review.
-
NRG1 Fusions: The New Kid on the Block.Curr Oncol Rep. 2025 Feb;27(2):190-194. doi: 10.1007/s11912-025-01640-y. Epub 2025 Jan 31. Curr Oncol Rep. 2025. PMID: 39888568 Review.
Cited by
-
Expert Consensus on the Diagnosis and Treatment of NRG1/2 Gene Fusion Solid Tumors.Glob Med Genet. 2024 Feb 27;11(1):86-99. doi: 10.1055/s-0044-1781457. eCollection 2024 Jan. Glob Med Genet. 2024. PMID: 38414979 Free PMC article. Review.
-
Developments in predictive biomarker testing and targeted therapy in advanced stage non-small cell lung cancer and their application across European countries.Lancet Reg Health Eur. 2024 Mar 1;38:100838. doi: 10.1016/j.lanepe.2024.100838. eCollection 2024 Mar. Lancet Reg Health Eur. 2024. PMID: 38476742 Free PMC article. Review.
-
Clinicopathological Characteristics of NRG1 Fusion-Positive Solid Tumors in Korean Patients.Cancer Res Treat. 2023 Oct;55(4):1087-1095. doi: 10.4143/crt.2023.682. Epub 2023 Jun 15. Cancer Res Treat. 2023. PMID: 37321274 Free PMC article. Review.
-
Neuregulin 1 Gene (NRG1). A Potentially New Targetable Alteration for the Treatment of Lung Cancer.Cancers (Basel). 2021 Oct 9;13(20):5038. doi: 10.3390/cancers13205038. Cancers (Basel). 2021. PMID: 34680187 Free PMC article. Review.
-
IBPGNET: lung adenocarcinoma recurrence prediction based on neural network interpretability.Brief Bioinform. 2024 Mar 27;25(3):bbae080. doi: 10.1093/bib/bbae080. Brief Bioinform. 2024. PMID: 38557672 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

