Oxytocin signal contributes to the adaptative growth of islets during gestation
- PMID: 34077390
- PMCID: PMC8240721
- DOI: 10.1530/EC-21-0043
Oxytocin signal contributes to the adaptative growth of islets during gestation
Abstract
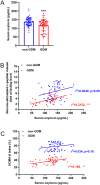
Background: Increased insulin production and secretion by pancreatic β-cells are important for ensuring the high insulin demand during gestation. However, the underlying mechanism of β-cell adaptation during gestation or gestational diabetes mellitus (GDM) remains unclear. Oxytocin is an important physiological hormone in gestation and delivery, and it also contributes to the maintenance of β-cell function. The aim of this study was to investigate the role of oxytocin in β-cell adaptation during pregnancy.
Methods: The relationship between the blood oxytocin level and pancreatic β-cell function in patients with GDM and healthy pregnant women was investigated. Gestating and non-gestating mice were used to evaluate the in vivo effect of oxytocin signal on β-cells during pregnancy. In vitro experiments were performed on INS-1 insulinoma cells.
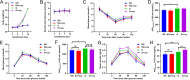
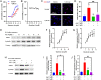
Results: The blood oxytocin levels were lower in patients with GDM than in healthy pregnant women and were associated with impaired pancreatic β-cell function. Acute administration of oxytocin increased insulin secretion in both gestating and non-gestating mice. A 3-week oxytocin treatment promoted the proliferation of pancreatic β-cells and increased the β-cell mass in gestating but not non-gestating mice. Antagonism of oxytocin receptors by atosiban impaired insulin secretion and induced GDM in gestating but not non-gestating mice. Oxytocin enhanced glucose-stimulated insulin secretion, activated the mitogen-activated protein kinase pathway, and promoted cell proliferation in INS-1 cells.
Conclusions: These findings provide strong evidence that oxytocin is needed for β-cell adaptation during pregnancy to maintain β-cell function, and the lack of oxytocin could be associated with the risk of GDM.
Keywords: gestational diabetes mellitus; insulin secretion; oxytocin; pancreatic β-cells; proliferation.
Figures






Similar articles
-
A mouse model of gestational glucose intolerance through exposure to a low protein diet during fetal and neonatal development.J Physiol. 2019 Aug;597(16):4237-4250. doi: 10.1113/JP277884. Epub 2019 Jul 11. J Physiol. 2019. PMID: 31206692
-
Enhanced PDGF signaling in gestational diabetes mellitus is involved in pancreatic β-cell dysfunction.Biochem Biophys Res Commun. 2019 Aug 20;516(2):402-407. doi: 10.1016/j.bbrc.2019.06.048. Epub 2019 Jun 17. Biochem Biophys Res Commun. 2019. PMID: 31217075
-
Oxytocin is present in islets and plays a role in beta-cell function and survival.Peptides. 2018 Feb;100:260-268. doi: 10.1016/j.peptides.2017.12.019. Epub 2017 Dec 20. Peptides. 2018. PMID: 29274352 Review.
-
miR-335-5p induces insulin resistance and pancreatic islet β-cell secretion in gestational diabetes mellitus mice through VASH1-mediated TGF-β signaling pathway.J Cell Physiol. 2019 May;234(5):6654-6666. doi: 10.1002/jcp.27406. Epub 2018 Oct 20. J Cell Physiol. 2019. PMID: 30341900
-
Gestational diabetes mellitus and subsequent development of overt diabetes mellitus.Dan Med Bull. 1998 Nov;45(5):495-509. Dan Med Bull. 1998. PMID: 9850811 Review.
Cited by
-
Research progress of placental vascular pathophysiological changes in pregnancy-induced hypertension and gestational diabetes mellitus.Front Physiol. 2022 Jul 19;13:954636. doi: 10.3389/fphys.2022.954636. eCollection 2022. Front Physiol. 2022. PMID: 35928561 Free PMC article. Review.
-
Impacts of Tocolytics on Maternal and Neonatal Glucose Levels in Women With Gestational Diabetes Mellitus.J Korean Med Sci. 2024 Sep 2;39(34):e236. doi: 10.3346/jkms.2024.39.e236. J Korean Med Sci. 2024. PMID: 39228183 Free PMC article.
-
Effects of oxytocin on the hair growth ability of dermal papilla cells.Sci Rep. 2023 Oct 20;13(1):15587. doi: 10.1038/s41598-023-40521-x. Sci Rep. 2023. PMID: 37863919 Free PMC article.
-
Oxytocin in Women's Health and Disease.Front Endocrinol (Lausanne). 2022 Feb 15;13:786271. doi: 10.3389/fendo.2022.786271. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35242106 Free PMC article. Review.
-
Potential Therapeutic Targeting Neurotransmitter Receptors in Diabetes.Front Endocrinol (Lausanne). 2022 May 20;13:884549. doi: 10.3389/fendo.2022.884549. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35669692 Free PMC article. Review.
References
-
- Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A, Ueta Y, Zingg HH, Chvatal A, Sykova E, et al. Review: Oxytocin: crossing the bridge between basic science and pharmacotherapy. CNS Neuroscience and Therapeutics 2010. 16 e138–e156. (10.1111/j.1755-5949.2010.00185.x) - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

