Cognitive deficits in episodic ataxia type 2 mouse models
- PMID: 34077522
- PMCID: PMC8444449
- DOI: 10.1093/hmg/ddab149
Cognitive deficits in episodic ataxia type 2 mouse models
Abstract
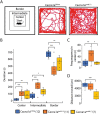
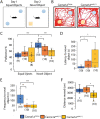
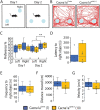
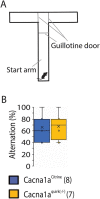
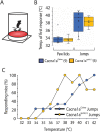
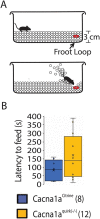
Episodic ataxia type 2 (EA2) is a rare autosomal dominant disorder characterized by motor incoordination, paroxysmal dystonia, vertigo, nystagmus and more recently cognitive deficits. To date over 100 mutations in the CACNA1A gene have been identified in EA2 patients leading to a loss of P/Q-type channel activity, dysfunction of cerebellar Purkinje cells and motor incoordination. To determine if the cerebellum is contributing to these cognitive deficits, we examined two different EA2 mouse models for cognition impairments where CACNA1A was removed specifically from cerebellar Purkinje or granule cells postnatally. Both mutant mouse models showed anxiolytic behavior to lighted, open areas in the open field and light/dark place preference tests but enhanced anxiousness in the novel suppressed feeding test. However, EA2 mice continued to show augmented latencies in the light/dark preference test and when the arena was divided into two dark zones in the dark/dark preference test. Moreover, increased latencies were also displayed in the novel object recognition test, indicating that EA2 mice are indecisive and anxious to explore new territories and objects and may have memory recognition deficits. Exposure to a foreign mouse led to deficiencies in attention and sniffing as well as in social and genital sniffing. These data suggest that postnatal removal of the P/Q type calcium channel from the cerebellum regulates neuronal activity involved in anxiety, memory, decision making and social interactions. Our EA2 mice will provide a model to identify the mechanisms and therapeutic agents underlying cognitive and psychiatric disorders seen in EA2 patients.
© The Author(s) 2021. Published by Oxford University Press.
Figures







Similar articles
-
The first knockin mouse model of episodic ataxia type 2.Exp Neurol. 2014 Nov;261:553-62. doi: 10.1016/j.expneurol.2014.08.001. Epub 2014 Aug 8. Exp Neurol. 2014. PMID: 25109669 Free PMC article.
-
Characterization of the dominant inheritance mechanism of Episodic Ataxia type 2.Neurobiol Dis. 2017 Oct;106:110-123. doi: 10.1016/j.nbd.2017.07.004. Epub 2017 Jul 5. Neurobiol Dis. 2017. PMID: 28688851
-
RNAi silencing of P/Q-type calcium channels in Purkinje neurons of adult mouse leads to episodic ataxia type 2.Neurobiol Dis. 2014 Aug;68:47-56. doi: 10.1016/j.nbd.2014.04.005. Epub 2014 Apr 21. Neurobiol Dis. 2014. PMID: 24768804
-
Episodic ataxia type 2: phenotype characteristics of a novel CACNA1A mutation and review of the literature.J Neurol. 2014 May;261(5):983-91. doi: 10.1007/s00415-014-7310-2. J Neurol. 2014. PMID: 24658662 Review.
-
Episodic ataxias 1 and 2.Handb Clin Neurol. 2012;103:595-602. doi: 10.1016/B978-0-444-51892-7.00042-5. Handb Clin Neurol. 2012. PMID: 21827920 Review.
Cited by
-
A chlorzoxazone-folic acid combination improves cognitive affective decline in SCA2-58Q mice.Sci Rep. 2023 Aug 3;13(1):12588. doi: 10.1038/s41598-023-39331-y. Sci Rep. 2023. PMID: 37537226 Free PMC article.
-
Intellectual Disability in Episodic Ataxia Type 2: Beyond Paroxysmal Vertigo and Ataxia.J Clin Neurol. 2024 Nov;20(6):563-570. doi: 10.3988/jcn.2024.0274. J Clin Neurol. 2024. PMID: 39505308 Free PMC article.
-
Memory decline, anxiety and depression in the mouse model of spinocerebellar ataxia type 3.Hum Mol Genet. 2024 Feb 1;33(4):299-317. doi: 10.1093/hmg/ddad179. Hum Mol Genet. 2024. PMID: 37862125 Free PMC article.
-
CACNA1A haploinsufficiency leads to reduced synaptic function and increased intrinsic excitability.Brain. 2025 Apr 3;148(4):1286-1301. doi: 10.1093/brain/awae330. Brain. 2025. PMID: 39460936 Free PMC article.
-
A combination of chlorzoxazone and folic acid improves recognition memory, anxiety and depression in SCA3-84Q mice.Hum Mol Genet. 2024 Aug 6;33(16):1406-1419. doi: 10.1093/hmg/ddae079. Hum Mol Genet. 2024. PMID: 38727562 Free PMC article.
References
-
- Jen, J., Kim, G.W. and Baloh, R.W. (2004) Clinical spectrum of episodic ataxia type 2. Neurology, 62, 17–22. - PubMed
-
- Nachbauer, W., Nocker, M., Karner, E., Stankovic, I., Unterberger, I., Eigentler, A., Schneider, R., Poewe, W., Delazer, M. and Boesch, S. (2014) Episodic ataxia type 2: phenotype characteristics of a novel CACNA1A mutation and review of the literature. J. Neurol., 261, 983–991. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

