Prognostic Role of Circulating Tumor Cells in Metastatic Renal Cell Carcinoma: A Large, Multicenter, Prospective Trial
- PMID: 34077597
- PMCID: PMC8417856
- DOI: 10.1002/onco.13842
Prognostic Role of Circulating Tumor Cells in Metastatic Renal Cell Carcinoma: A Large, Multicenter, Prospective Trial
Abstract
Background: Circulating tumor cells (CTCs) correlate with adverse prognosis in patients with breast, colorectal, lung, and prostate cancer. Little data are available for renal cell carcinoma (RCC).
Materials and methods: We designed a multicenter prospective observational study to assess the correlation between CTC counts and progression-free survival (PFS) in patients with metastatic RCC treated with an antiangiogenic tyrosine kinase inhibitor as a first-line regimen; overall survival (OS) and response were secondary objectives. CTC counts were enumerated by the CellSearch system at four time points: day 0 of treatment, day 28, day 56 and then at progression, or at 12 months in the absence of progression.
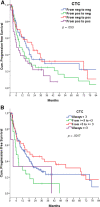
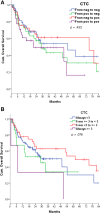
Results: One hundred ninety-five eligible patients with a median age of 69 years were treated with sunitinib (77.5%) or pazopanib (21%). At baseline, 46.7% of patients had one or more CTCs per milliliter (range, 1 to 263). Thirty patients had at least three CTCs, with a median PFS of 5.8 versus 15 months in the remaining patients (p = .002; hazard ratio [HR], 1.99), independently of the International Metastatic RCC Database Consortium score at multivariate analysis (HR, 1.91; 95% confidence interval [CI], 1.16-3.14). Patients with at least three CTCs had a shorter estimated OS of 13.8 months versus 52.8 months in those with fewer than three CTCs (p = .003; HR, 1.99; multivariate analysis HR, 1.67; 95% CI, 0.95-2.93). Baseline CTC counts did not correlate with response; neither did having CTC sequencing counts greater than or equal to one, two, three, four, or five.
Conclusion: We provide prospective evidence that the presence of three or more CTCs at baseline is associated with a significantly shorter PFS and OS in patients with metastatic RCC.
Implications for practice: This prospective study evaluated whether the presence of circulating tumor cells (CTCs) in the peripheral blood correlates with activity of first-line tyrosine kinase inhibitors in metastatic renal cell carcinoma (RCC). This study demonstrated that almost half of patients with metastatic RCC have at least one CTC in their blood and that those patients with at least three CTCs are at increased risk of early progressive disease and early death due to RCC. Studies incorporating CTC counts in the prognostic algorithms of metastatic RCC are warranted.
Keywords: Biomarker; Circulating tumor cells; Metastases; Pazopanib; Renal cell carcinoma; Sunitinib.
© 2021 AlphaMed Press.
Conflict of interest statement
Figures


References
-
- Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal‐cell carcinoma. N Engl J Med 2017;376:354–366. - PubMed
-
- Rini BI, Plimack ER, Stus V et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1116–1127. - PubMed
-
- Heng DY, Xie W, Regan MM et al. Prognostic factors for overall survival in patients with metastatic renalcell carcinoma treated with vascular endothelial growth factor‐targeted agents: Results from a large, multicenter study. J Clin Oncol 2009;27:5794–5799. - PubMed
-
- Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

