Microbial exposure during early human development primes fetal immune cells
- PMID: 34077752
- PMCID: PMC8240556
- DOI: 10.1016/j.cell.2021.04.039
Microbial exposure during early human development primes fetal immune cells
Abstract
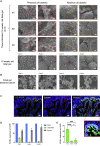
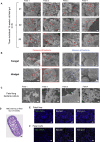
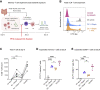
The human fetal immune system begins to develop early during gestation; however, factors responsible for fetal immune-priming remain elusive. We explored potential exposure to microbial agents in utero and their contribution toward activation of memory T cells in fetal tissues. We profiled microbes across fetal organs using 16S rRNA gene sequencing and detected low but consistent microbial signal in fetal gut, skin, placenta, and lungs in the 2nd trimester of gestation. We identified several live bacterial strains including Staphylococcus and Lactobacillus in fetal tissues, which induced in vitro activation of memory T cells in fetal mesenteric lymph node, supporting the role of microbial exposure in fetal immune-priming. Finally, using SEM and RNA-ISH, we visualized discrete localization of bacteria-like structures and eubacterial-RNA within 14th weeks fetal gut lumen. These findings indicate selective presence of live microbes in fetal organs during the 2nd trimester of gestation and have broader implications toward the establishment of immune competency and priming before birth.
Keywords: Tem; Treg; bacteria; fetal Development; fetal immunity; immune memory; immune priming; microbes; microbiome.
Crown Copyright © 2021. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures










Comment in
-
Bacteria make T cell memories in utero.Cell. 2021 Jun 24;184(13):3356-3357. doi: 10.1016/j.cell.2021.05.044. Cell. 2021. PMID: 34171317
-
Over-celling fetal microbial exposure.Cell. 2021 Nov 24;184(24):5839-5841. doi: 10.1016/j.cell.2021.10.026. Cell. 2021. PMID: 34822779 No abstract available.
-
Reply to Over-celling fetal microbial exposure.Cell. 2021 Nov 24;184(24):5842-5844. doi: 10.1016/j.cell.2021.10.028. Cell. 2021. PMID: 34822780 No abstract available.
References
-
- Abdelfattah A., Wisniewski M., Schena L., Tack A.J.M. Experimental evidence of microbial inheritance in plants and transmission routes from seed to phyllosphere and root. Environ. Microbiol. 2021;23:2199–2214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

