Neurovascular crosstalk coordinates the central nervous system development
- PMID: 34077852
- PMCID: PMC8411665
- DOI: 10.1016/j.conb.2021.04.005
Neurovascular crosstalk coordinates the central nervous system development
Abstract
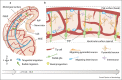
Purpose of the review: The synchronic development of vascular and nervous systems is orchestrated by common molecules that regulate the communication between both systems. The identification of these common guiding cues and the developmental processes regulated by neurovascular communication are slowly emerging. In this review, we describe the molecules modulating the neurovascular development and their impact in processes such as angiogenesis, neurogenesis, neuronal migration, and brain homeostasis.
Recent findings: Blood vessels not only are involved in nutrient and oxygen supply of the central nervous system (CNS) but also exert instrumental functions controlling developmental neurogenesis, CNS cytoarchitecture, and neuronal plasticity. Conversely, neurons modulate CNS vascularization and brain endothelial properties such as blood-brain barrier and vascular hyperemia.
Summary: The integration of the active role of endothelial cells in the development and maintenance of neuronal function is important to obtain a more holistic view of the CNS complexity and also to understand how the vasculature is involved in neuropathological conditions.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures

References
-
- Hogan K.A., Ambler C.A., Chapman D.L., Bautch V.L. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development. 2004;131:1503–1513. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

