Regulation of the Methylation and Expression Levels of the BMPR2 Gene by SIN3a as a Novel Therapeutic Mechanism in Pulmonary Arterial Hypertension
- PMID: 34078089
- PMCID: PMC8293289
- DOI: 10.1161/CIRCULATIONAHA.120.047978
Regulation of the Methylation and Expression Levels of the BMPR2 Gene by SIN3a as a Novel Therapeutic Mechanism in Pulmonary Arterial Hypertension
Abstract
Background: Epigenetic mechanisms are critical in the pathogenesis of pulmonary arterial hypertension (PAH). Previous studies have suggested that hypermethylation of the BMPR2 (bone morphogenetic protein receptor type 2) promoter is associated with BMPR2 downregulation and progression of PAH. Here, we investigated for the first time the role of SIN3a (switch-independent 3a), a transcriptional regulator, in the epigenetic mechanisms underlying hypermethylation of BMPR2 in the pathogenesis of PAH.
Methods: We used lung samples from PAH patients and non-PAH controls, preclinical mouse and rat PAH models, and human pulmonary arterial smooth muscle cells. Expression of SIN3a was modulated using a lentiviral vector or a siRNA in vitro and a specific adeno-associated virus serotype 1 or a lentivirus encoding for human SIN3a in vivo.
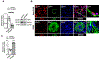
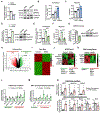
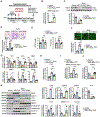
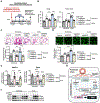
Results: SIN3a is a known transcriptional regulator; however, its role in cardiovascular diseases, especially PAH, is unknown. It is interesting that we detected a dysregulation of SIN3 expression in patients and in rodent models, which is strongly associated with decreased BMPR2 expression. SIN3a is known to regulate epigenetic changes. Therefore, we tested its role in the regulation of BMPR2 and found that BMPR2 is regulated by SIN3a. It is interesting that SIN3a overexpression inhibited human pulmonary arterial smooth muscle cells proliferation and upregulated BMPR2 expression by preventing the methylation of the BMPR2 promoter region. RNA-sequencing analysis suggested that SIN3a downregulated the expression of DNA and histone methyltransferases such as DNMT1 (DNA methyltransferase 1) and EZH2 (enhancer of zeste 2 polycomb repressive complex 2) while promoting the expression of the DNA demethylase TET1 (ten-eleven translocation methylcytosine dioxygenase 1). Mechanistically, SIN3a promoted BMPR2 expression by decreasing CTCF (CCCTC-binding factor) binding to the BMPR2 promoter. Last, we identified intratracheal delivery of adeno-associated virus serotype human SIN3a to be a beneficial therapeutic approach in PAH by attenuating pulmonary vascular and right ventricle remodeling, decreasing right ventricle systolic pressure and mean pulmonary arterial pressure, and restoring BMPR2 expression in rodent models of PAH.
Conclusions: All together, our study unveiled the protective and beneficial role of SIN3a in pulmonary hypertension. We also identified a novel and distinct molecular mechanism by which SIN3a regulates BMPR2 in human pulmonary arterial smooth muscle cells. Our study also identified lung-targeted SIN3a gene therapy using adeno-associated virus serotype 1 as a new promising therapeutic strategy for treating patients with PAH.
Keywords: DNA; dependovirus; epigenesis, genetic; genetic therapy; hypertension; methylation; pulmonary; pulmonary arterial hypertension.
Conflict of interest statement
Disclosures
The authors report no conflicts.
Figures




References
-
- Montani D, Girerd B, Gunther S, Riant F, Tournier-Lasserve E, Magy L, Maazi N, Guignabert C, Savale L, Sitbon O, Simonneau G, Soubrier F and Humbert M. Pulmonary arterial hypertension in familial hemiplegic migraine with ATP1A2 channelopathy. Eur Respir J. 2014;43:641–3. - PubMed
-
- Montani D, Chaumais MC, Guignabert C, Gunther S, Girerd B, Jais X, Algalarrondo V, Price LC, Savale L, Sitbon O, Simonneau G and Humbert M. Targeted therapies in pulmonary arterial hypertension. Pharmacol Ther. 2014;141:172–91. - PubMed
-
- Thompson AAR and Lawrie A. Targeting Vascular Remodeling to Treat Pulmonary Arterial Hypertension. Trends Mol Med. 2017;23:31–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

