Real-life use of onabotulinumtoxinA reduces healthcare resource utilization in individuals with chronic migraine: the REPOSE study
- PMID: 34078259
- PMCID: PMC8173963
- DOI: 10.1186/s10194-021-01260-4
Real-life use of onabotulinumtoxinA reduces healthcare resource utilization in individuals with chronic migraine: the REPOSE study
Abstract
Background: Chronic migraine (CM) is associated with substantial economic burden. Real-world data suggests that onabotulinumtoxinA treatment for CM reduces healthcare resource utilisation (HRU) and related costs.
Methods: REPOSE was a 2-year prospective, multicentre, non-interventional, observational study to describe the real-world use of onabotulinumtoxinA in adult patients with CM. This analysis examined the impact of onabotulinumtoxinA on HRU. Patients received onabotulinumtoxinA treatment approximately every 12 weeks according to their physicians' discretion, guided by the summary of product characteristics (SPC) and PREEMPT injection paradigm. HRU outcome measures were collected at baseline and all administration visits and included headache-related hospitalizations and healthcare professional (HCP) visits. Health economic data, including family doctor and specialist visits, inpatient treatment for headache, acupuncture, technical diagnostics, use of nonpharmacologic remedies, and work productivity were also collected for patients enrolled at German study centres.
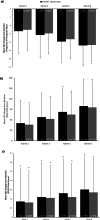
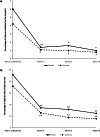
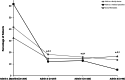
Results: Overall, 641 patients were enrolled at 78 study centres across 7 countries (Germany, UK, Italy, Spain, Norway, Sweden, and Russia), 633 received ≥1 onabotulinumtoxinA dose, and 128 completed the 2-year study. Patients were, on average, aged 45 years, 85% were female, and 60% (n = 377) were from Germany. At the end of the 2-year observation period, significantly fewer patients reported headache-related hospitalizations (p < 0.02) and HCP visits (p < 0.001) within the past 3 months than in the 3 months before baseline. In the German population, reductions were observed across all health services at all follow-up visits compared with baseline. The percentage of patients who saw a family doctor decreased from 41.7% at baseline to 13.5% at administration visit 8 and visits to a medical specialist decreased from 61.7% to 5.2% of patients. Inpatient acute treatment and technical diagnostics declined from 6.4% and 19.7% of patients at baseline to 0.0% and 1.0% at administration 8, respectively. The use of nonpharmacologic remedies and medication for the acute treatment of migraine also decreased with continued onabotulinumtoxinA treatment. Work incapacity, disability, absenteeism, and impaired performance at school/work improved with onabotulinumtoxinA treatment for CM over the 2-year observation period.
Conclusions: Real-world evidence from REPOSE demonstrates that onabotulinumtoxinA treatment is associated with decreased HRU and supports the long-term benefits associated with the use of onabotulinumtoxinA for CM in clinical practice.
Trial registration: NCT01686581 . Name of registry: ClinicalTrials.gov. URL of registry: Date of retrospective registration: September 18, 2012. Date of enrolment of first patient: July 23, 2012.
Keywords: Burden; Chronic migraine; Economic; Headache; Healthcare; Healthcare resource utilization; OnabotulinumtoxinA.
Conflict of interest statement
Financial arrangements of the authors with companies whose products may be related to the present report are listed below, as declared by the authors.
Figures

Similar articles
-
Real-World Safety and Efficacy of 156 U - 195 U OnabotulinumtoxinA in Adults With Chronic Migraine: Results From the REPOSE Study.BMC Neurol. 2025 May 6;25(1):197. doi: 10.1186/s12883-025-04087-7. BMC Neurol. 2025. PMID: 40329224 Free PMC article.
-
An open-label prospective study of the real-life use of onabotulinumtoxinA for the treatment of chronic migraine: the REPOSE study.J Headache Pain. 2019 Mar 7;20(1):26. doi: 10.1186/s10194-019-0976-1. J Headache Pain. 2019. PMID: 30845917 Free PMC article.
-
Real-life use of onabotulinumtoxinA for symptom relief in patients with chronic migraine: REPOSE study methodology and baseline data.J Headache Pain. 2017 Sep 6;18(1):93. doi: 10.1186/s10194-017-0802-6. J Headache Pain. 2017. PMID: 28879545 Free PMC article.
-
OnabotulinumtoxinA injection in the treatment of chronic migraine.Prog Brain Res. 2020;255:171-206. doi: 10.1016/bs.pbr.2020.05.013. Epub 2020 Jun 30. Prog Brain Res. 2020. PMID: 33008506 Review.
-
OnabotulinumtoxinA: Discussion of the evidence for effectiveness of OnabotulinumA and its place in chronic migraine treatment.Handb Clin Neurol. 2024;199:87-106. doi: 10.1016/B978-0-12-823357-3.00007-0. Handb Clin Neurol. 2024. PMID: 38307674 Review.
Cited by
-
Insights from 25 years of onabotulinumtoxinA in migraine - mechanisms and management.Nat Rev Neurol. 2024 Sep;20(9):555-568. doi: 10.1038/s41582-024-01002-5. Epub 2024 Aug 19. Nat Rev Neurol. 2024. PMID: 39160284 Review.
-
Real-World Safety and Efficacy of 156 U - 195 U OnabotulinumtoxinA in Adults With Chronic Migraine: Results From the REPOSE Study.BMC Neurol. 2025 May 6;25(1):197. doi: 10.1186/s12883-025-04087-7. BMC Neurol. 2025. PMID: 40329224 Free PMC article.
-
A Retrospective Real-Life Multicenter Study on Concurrent Oral Preventive Treatments in Patients with Chronic Migraine Treated with OnabotulinumtoxinA.CNS Drugs. 2023 May;37(5):453-465. doi: 10.1007/s40263-023-01001-y. Epub 2023 May 22. CNS Drugs. 2023. PMID: 37212943 Free PMC article.
-
OnabotulinumtoxinA Treatment in Chronic Migraine: Investigation of Its Effects on Disability, Headache and Neck Pain Intensity.Toxins (Basel). 2022 Dec 30;15(1):29. doi: 10.3390/toxins15010029. Toxins (Basel). 2022. PMID: 36668849 Free PMC article.
-
Real-world experience of OnabotulinumtoxinA treatment in female patients with chronic migraine: a qualitative study using in-depth interviews.Ann Med. 2023;55(2):2255215. doi: 10.1080/07853890.2023.2255215. Ann Med. 2023. PMID: 37708876 Free PMC article.
References
-
- Yoon MS, Manack A, Schramm S, Fritsche G, Obermann M, Diener HC, Moebus S, Katsarava Z. Chronic migraine and chronic tension-type headache are associated with concomitant low back pain: results of the German headache consortium study. Pain. 2013;154(3):484–492. doi: 10.1016/j.pain.2012.12.010. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

