Promoting HIV indicator condition-guided testing in hospital settings (PROTEST 2.0): study protocol for a multicentre interventional study
- PMID: 34078315
- PMCID: PMC8173796
- DOI: 10.1186/s12879-021-06183-8
Promoting HIV indicator condition-guided testing in hospital settings (PROTEST 2.0): study protocol for a multicentre interventional study
Abstract
Background: Late presentation remains a key barrier towards controlling the HIV epidemic. Indicator conditions (ICs) are those that are AIDS-defining, associated with a prevalence of undiagnosed HIV > 0.1%, or whose clinical management would be impeded if an HIV infection were undiagnosed. IC-guided HIV testing is an effective strategy in identifying undiagnosed HIV, but opportunities for earlier HIV diagnosis through IC-guided testing are being missed. We present a protocol for an interventional study to improve awareness of IC-guided testing and increase HIV testing in patients presenting with ICs in a hospital setting.
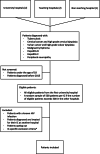
Methods: We designed a multicentre interventional study to be implemented at five hospitals in the region of Amsterdam, the Netherlands. Seven ICs were selected for which HIV test ratios (proportion of patients with an IC tested for HIV) will be measured: tuberculosis, cervical/vulvar cancer or high-grade cervical/vulvar dysplasia, malignant lymphoma, hepatitis B and C, and peripheral neuropathy. Prior to the intervention, a baseline assessment of HIV test ratios across ICs will be performed in eligible patients (IC diagnosed January 2015 through May 2020, ≥18 years, not known HIV positive) and an assessment of barriers and facilitators for HIV testing amongst relevant specialties will be conducted using qualitative (interviews) and quantitative methods (questionnaires). The intervention phase will consist of an educational intervention, including presentation of baseline results as competitive graphical audit and feedback combined with discussion on implementation and opportunities for improvement. The effect of the intervention will be assessed by comparing HIV test ratios of the pre-intervention and post-intervention periods. The primary endpoint is the HIV test ratio within ±3 months of IC diagnosis. Secondary endpoints are the HIV test ratio within ±6 months of diagnosis, ratio ever tested for HIV, HIV positivity percentage, proportion of late presenters and proportion with known HIV status prior to initiating treatment for their IC.
Discussion: This protocol presents a strategy aimed at increasing awareness of the benefits of IC-guided testing and increasing HIV testing in patients presenting with ICs in hospital settings to identify undiagnosed HIV in Amsterdam, the Netherlands.
Trial registration: Dutch trial registry: NL7521 . Registered 14 February 2019.
Keywords: HIV testing; Healthcare quality improvement; Implementation; Indicator condition; Multifaceted intervention.
Conflict of interest statement
AB is a member of the editorial board of BMC infectious diseases. All other authors declare that they have no competing interests directly related to this study.
Figures
References
-
- UNAIDS . Global HIV & AIDS statistics — 2020 fact sheet. 2020.
-
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR, HPTN 052 Study Team Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- P-42702/Aids Fonds
- Aids Fonds (grant number: 2013169), Stichting Amsterdam Dinner Foundation, Bristol-Myers Squibb International Corp. (study number: AI424-541), Gilead Sciences Europe Ltd (grant number: PA-HIV-PREP-16-0024), Gilead Sciences (protocol numbers: CO-NL-276-4222, CO-US-276-1712), Janssen Pharmaceutica (reference number: PHNL/JAN/0714/0005b/1912fde), M.A.C AIDS Fund, ViiV Healthcare (PO numbers: 3000268822, 3000747780) and ZonMw (grant number: 522002003)./H-TEAM initiative
LinkOut - more resources
Full Text Sources
Medical