Sexual conflict drives micro- and macroevolution of sexual dimorphism in immunity
- PMID: 34078377
- PMCID: PMC8170964
- DOI: 10.1186/s12915-021-01049-6
Sexual conflict drives micro- and macroevolution of sexual dimorphism in immunity
Abstract
Background: Sexual dimorphism in immunity is believed to reflect sex differences in reproductive strategies and trade-offs between competing life history demands. Sexual selection can have major effects on mating rates and sex-specific costs of mating and may thereby influence sex differences in immunity as well as associated host-pathogen dynamics. Yet, experimental evidence linking the mating system to evolved sexual dimorphism in immunity are scarce and the direct effects of mating rate on immunity are not well established. Here, we use transcriptomic analyses, experimental evolution and phylogenetic comparative methods to study the association between the mating system and sexual dimorphism in immunity in seed beetles, where mating causes internal injuries in females.
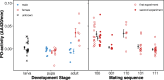
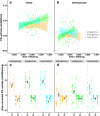
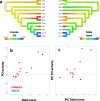
Results: We demonstrate that female phenoloxidase (PO) activity, involved in wound healing and defence against parasitic infections, is elevated relative to males. This difference is accompanied by concomitant sex differences in the expression of genes in the prophenoloxidase activating cascade. We document substantial phenotypic plasticity in female PO activity in response to mating and show that experimental evolution under enforced monogamy (resulting in low remating rates and reduced sexual conflict relative to natural polygamy) rapidly decreases female (but not male) PO activity. Moreover, monogamous females had evolved increased tolerance to bacterial infection unrelated to mating, implying that female responses to costly mating may trade off with other aspects of immune defence, an hypothesis which broadly accords with the documented sex differences in gene expression. Finally, female (but not male) PO activity shows correlated evolution with the perceived harmfulness of male genitalia across 12 species of seed beetles, suggesting that sexual conflict has a significant influence on sexual dimorphisms in immunity in this group of insects.
Conclusions: Our study provides insights into the links between sexual conflict and sexual dimorphism in immunity and suggests that selection pressures moulded by mating interactions can lead to a sex-specific mosaic of immune responses with important implications for host-pathogen dynamics in sexually reproducing organisms.
Keywords: Callosobruchus maculatus; Experimental evolution; Immunity; Mating; Phenoloxidase; Sexual conflict; Sexual dimorphism; Sexual selection; Sexually transmitted disease; Trade-off.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Oliva M, Muñoz-Aguirre M, Kim-Hellmuth S, Wucher V, Gewirtz ADH, Cotter DJ, Parsana P, Kasela S, Balliu B, Viñuela A, Castel SE, Mohammadi P, Aguet F, Zou Y, Khramtsova EA, Skol AD, Garrido-Martín D, Reverter F, Brown A, Evans P, Gamazon ER, Payne A, Bonazzola R, Barbeira AN, Hamel AR, Martinez-Perez A, Soria JM, GTEx Consortium§. Pierce BL, Stephens M, Eskin E, Dermitzakis ET, Segrè AV, Im HK, Engelhardt BE, Ardlie KG, Montgomery SB, Battle AJ, Lappalainen T, Guigó R, Stranger BE. The impact of sex on gene expression across human tissues. Science. 2020;369(6509):eaba3066. doi: 10.1126/science.aba3066. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

