The European Rare Kidney Disease Registry (ERKReg): objectives, design and initial results
- PMID: 34078418
- PMCID: PMC8173879
- DOI: 10.1186/s13023-021-01872-8
The European Rare Kidney Disease Registry (ERKReg): objectives, design and initial results
Abstract
Background: The European Rare Kidney Disease Reference Network (ERKNet) recently established ERKReg, a Web-based registry for all patients with rare kidney diseases. The main objectives of this core registry are to generate epidemiological information, identify current patient cohort for clinical research, explore diagnostic and therapeutic management practices, and monitor treatment performance and patient's outcomes. The registry has a modular design that allows to integrate comprehensive disease-specific registries as extensions to the core database. The diagnosis (Orphacode) and diagnostic information (clinical, imaging, histopathological, biochemical, immunological and genetic) are recorded. Anthropometric, kidney function, and disease-specific management and outcome items informing a set of 61 key performance indicators (KPIs) are obtained annually. Data quality is ensured by automated plausibility checks upon data entry and regular offline database checks prompting queries. Centre KPI statistics and benchmarking are calculated automatically.
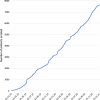
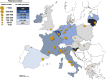
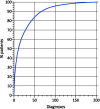
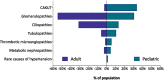
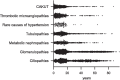
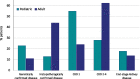
Results: Within the first 24 months since its launch, 7607 patients were enrolled to the registry at 45 pediatric and 12 specialized adult nephrology units from 21 countries. A kidney disease diagnosis had been established in 97.1% of these patients at time of enrolment. While 199 individual disease entities were reported by Orphacode, 50% of the cohort could be classified with 11, 80% with 43 and 95% with 92 codes. Two kidney diagnoses were assigned in 6.5% of patients; 5.9% suffered from syndromic disease. Whereas glomerulopathies (54.8%) and ciliopathies including autosomal dominant polycystic kidney disease (ADPKD) (31.5%) were the predominant disease groups among adults, the pediatric disease spectrum encompassed congenital anomalies of the kidney and urinary tract (CAKUT) (33.7%), glomerulopathies (30.7%), ciliopathies (14.0%), tubulopathies (9.2%), thrombotic microangiopathies (5.6%), and metabolic nephropathies (4.1%). Genetically confirmed diagnoses were reported in 24% of all pediatric and 12% adult patients, whereas glomerulopathies had been confirmed by kidney biopsy in 80.4% adult versus 38.5% pediatric glomerulopathy cases.
Conclusions: ERKReg is a rapidly growing source of epidemiological information and patient cohorts for clinical research, and an innovative tool to monitor management quality and patient outcomes.
Keywords: Epidemiology; European Rare Kidney Disease Reference Network (ERKNet); Nephrology; Pediatric nephrology; Registry.
Conflict of interest statement
The authors declare that they have no competing interests with regards to the data presented in this manuscript.
Figures


References
-
- European Rare Kidney Disease Reference Network (ERKNet). 2017. https://erknet.org/index.php?id=home. Accessed 27 Jan 2021.

