Effect of dexamethasone on complications or all cause mortality after major non-cardiac surgery: multicentre, double blind, randomised controlled trial
- PMID: 34078591
- PMCID: PMC8171383
- DOI: 10.1136/bmj.n1162
Effect of dexamethasone on complications or all cause mortality after major non-cardiac surgery: multicentre, double blind, randomised controlled trial
Abstract
Objective: To assess the effect of dexamethasone on complications or all cause mortality after major non-cardiac surgery.
Design: Phase III, randomised, double blind, placebo controlled trial.
Setting: 34 centres in France, December 2017 to March 2019.
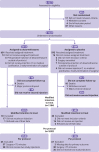
Participants: 1222 adults (>50 years) requiring major non-cardiac surgery with an expected duration of more than 90 minutes. The anticipated time frame for recruitment was 24 months.
Interventions: Participants were randomised to receive either dexamethasone (0.2 mg/kg immediately after the surgical procedure, and on day 1) or placebo. Randomisation was stratified on the two prespecified criteria of cancer and thoracic procedure.
Main outcomes measures: The primary outcome was a composite of postoperative complications or all cause mortality within 14 days after surgery, assessed in the modified intention-to-treat population (at least one treatment administered).
Results: Of the 1222 participants who underwent randomisation, 1184 (96.9%) were included in the modified intention-to-treat population. 14 days after surgery, 101 of 595 participants (17.0%) in the dexamethasone group and 117 of 589 (19.9%) in the placebo group had complications or died (adjusted odds ratio 0.81, 95% confidence interval 0.60 to 1.08; P=0.15). In the stratum of participants who underwent non-thoracic surgery (n=1038), the primary outcome occurred in 69 of 520 participants (13.3%) in the dexamethasone group and 93 of 518 (18%) in the placebo group (adjusted odds ratio 0.70, 0.50 to 0.99). Adverse events were reported in 288 of 613 participants (47.0%) in the dexamethasone group and 296 of 609 (48.6%) in the placebo group (P=0.46).
Conclusions: Dexamethasone was not found to significantly reduce the incidence of complications and death in patients 14 days after major non-cardiac surgery. The 95% confidence interval for the main result was, however, wide and suggests the possibility of important clinical effectiveness.
Trial registration: ClinicalTrials.gov NCT03218553.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the French Ministry of Health and Nantes University Hospital for the submitted work; KA reports receiving lecture fees from Baxter, Fisher and Paykel, and LFB and consulting fees from Edward Lifesciences, LFB (Laboratoire français du fractionnement et des biotechnologies). MB reports receiving consulting fees from Becton Dickinson. SJ reports receiving personal fees from Drager, Medtronic, Baxter, Medtronic, Fisher & Paykel, and Fresenius-Xenios. ML reports receiving consulting fees from Aspen, Orion, MSD, Pfizer, 3M, Octapharma, and Edwards Lifesciences. EF reports receiving consulting fees from Drager Medical, GE Healthcare, Edwards Lifesciences, and Orion Pharma, and lectures fees from Fresenius Kabi, Baxter, and Fisher & Paykel. AR reports receiving consulting fees from Merck and bioMerieux.
Figures

References
-
- Bainbridge D, Martin J, Arango M, Cheng D, Evidence-based Peri-operative Clinical Outcomes Research (EPiCOR) Group . Perioperative and anaesthetic-related mortality in developed and developing countries: a systematic review and meta-analysis. Lancet 2012;380:1075-81. 10.1016/S0140-6736(12)60990-8 - DOI - PubMed
-
- Peden CJ, Stephens T, Martin G, et al. Enhanced Peri-Operative Care for High-risk patients (EPOCH) trial group . Effectiveness of a national quality improvement programme to improve survival after emergency abdominal surgery (EPOCH): a stepped-wedge cluster-randomised trial. Lancet 2019;393:2213-21. 10.1016/S0140-6736(18)32521-2 - DOI - PubMed
-
- Pearse RM, Harrison DA, MacDonald N, et al. OPTIMISE Study Group . Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 2014;311:2181-90. 10.1001/jama.2014.5305 - DOI - PubMed
-
- Pearse RM, Moreno RP, Bauer P, et al. European Surgical Outcomes Study (EuSOS) group for the Trials groups of the European Society of Intensive Care Medicine and the European Society of Anaesthesiology . Mortality after surgery in Europe: a 7 day cohort study. Lancet 2012;380:1059-65. 10.1016/S0140-6736(12)61148-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
