Vorasidenib, a Dual Inhibitor of Mutant IDH1/2, in Recurrent or Progressive Glioma; Results of a First-in-Human Phase I Trial
- PMID: 34078652
- PMCID: PMC8364866
- DOI: 10.1158/1078-0432.CCR-21-0611
Vorasidenib, a Dual Inhibitor of Mutant IDH1/2, in Recurrent or Progressive Glioma; Results of a First-in-Human Phase I Trial
Abstract
Purpose: Lower grade gliomas (LGGs) are malignant brain tumors. Current therapy is associated with short- and long-term toxicity. Progression to higher tumor grade is associated with contrast enhancement on MRI. The majority of LGGs harbor mutations in the genes encoding isocitrate dehydrogenase 1 or 2 (IDH1/IDH2). Vorasidenib (AG-881) is a first-in-class, brain-penetrant, dual inhibitor of the mutant IDH1 and mutant IDH2 enzymes.
Patients and methods: We conducted a multicenter, open-label, phase I, dose-escalation study of vorasidenib in 93 patients with mutant IDH1/2 (mIDH1/2) solid tumors, including 52 patients with glioma that had recurred or progressed following standard therapy. Vorasidenib was administered orally, once daily, in 28-day cycles until progression or unacceptable toxicity. Enrollment is complete; this trial is registered with ClinicalTrials.gov, NCT02481154.
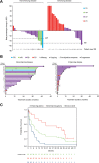
Results: Vorasidenib showed a favorable safety profile in the glioma cohort. Dose-limiting toxicities of elevated transaminases occurred at doses ≥100 mg and were reversible. The protocol-defined objective response rate per Response Assessment in Neuro-Oncology criteria for LGG in patients with nonenhancing glioma was 18% (one partial response, three minor responses). The median progression-free survival was 36.8 months [95% confidence interval (CI), 11.2-40.8] for patients with nonenhancing glioma and 3.6 months (95% CI, 1.8-6.5) for patients with enhancing glioma. Exploratory evaluation of tumor volumes in patients with nonenhancing glioma showed sustained tumor shrinkage in multiple patients.
Conclusions: Vorasidenib was well tolerated and showed preliminary antitumor activity in patients with recurrent or progressive nonenhancing mIDH LGG.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
- Clin Cancer Res. 27:4457.
References
-
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803–20. - PubMed
-
- van den Bent MJ, Smits M, Kros JM, Chang SM. Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol 2017;35:2394–401. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

