Bud endodormancy in deciduous fruit trees: advances and prospects
- PMID: 34078882
- PMCID: PMC8172858
- DOI: 10.1038/s41438-021-00575-2
Bud endodormancy in deciduous fruit trees: advances and prospects
Abstract
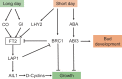
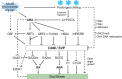
Bud endodormancy is a complex physiological process that is indispensable for the survival, growth, and development of deciduous perennial plants. The timely release of endodormancy is essential for flowering and fruit production of deciduous fruit trees. A better understanding of the mechanism of endodormancy will be of great help in the artificial regulation of endodormancy to cope with climate change and in creating new cultivars with different chilling requirements. Studies in poplar have clarified the mechanism of vegetative bud endodormancy, but the endodormancy of floral buds in fruit trees needs further study. In this review, we focus on the molecular regulation of endodormancy induction, maintenance and release in floral buds of deciduous fruit trees. We also describe recent advances in quantitative trait loci analysis of chilling requirements in fruit trees. We discuss phytohormones, epigenetic regulation, and the detailed molecular network controlling endodormancy, centered on SHORT VEGETATIVE PHASE (SVP) and Dormancy-associated MADS-box (DAM) genes during endodormancy maintenance and release. Combining previous studies and our observations, we propose a regulatory model for bud endodormancy and offer some perspectives for the future.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Deciphering the genetic mechanisms of chilling requirement for bud endodormancy release in deciduous fruit trees.Mol Breed. 2024 Oct 8;44(10):70. doi: 10.1007/s11032-024-01510-8. eCollection 2024 Oct. Mol Breed. 2024. PMID: 39391168
-
I Want to (Bud) Break Free: The Potential Role of DAM and SVP-Like Genes in Regulating Dormancy Cycle in Temperate Fruit Trees.Front Plant Sci. 2019 Jan 10;9:1990. doi: 10.3389/fpls.2018.01990. eCollection 2018. Front Plant Sci. 2019. PMID: 30687377 Free PMC article. Review.
-
VvDAM-SVPs genes are regulated by FLOWERING LOCUS T (VvFT) and not by ABA/low temperature-induced VvCBFs transcription factors in grapevine buds.Planta. 2021 Jan 12;253(2):31. doi: 10.1007/s00425-020-03561-5. Planta. 2021. PMID: 33438039
-
Elucidation of molecular and hormonal background of early growth cessation and endodormancy induction in two contrasting Populus hybrid cultivars.BMC Plant Biol. 2021 Feb 24;21(1):111. doi: 10.1186/s12870-021-02828-7. BMC Plant Biol. 2021. PMID: 33627081 Free PMC article.
-
Advancing Endodormancy Release in Temperate Fruit Trees Using Agrochemical Treatments.Front Plant Sci. 2022 Jan 14;12:812621. doi: 10.3389/fpls.2021.812621. eCollection 2021. Front Plant Sci. 2022. PMID: 35111185 Free PMC article. Review.
Cited by
-
Stable water isotopes reveal the onset of bud dormancy in temperate trees, whereas water content is a better proxy for dormancy release.Tree Physiol. 2024 Apr 3;44(4):tpae028. doi: 10.1093/treephys/tpae028. Tree Physiol. 2024. PMID: 38417929 Free PMC article.
-
Integrated proteomic, transcriptomic, and metabolomic profiling reveals that the gibberellin-abscisic acid hub runs flower development in the Chinese orchid Cymbidium sinense.Hortic Res. 2024 Mar 12;11(5):uhae073. doi: 10.1093/hr/uhae073. eCollection 2024 May. Hortic Res. 2024. PMID: 38738212 Free PMC article.
-
Molecular Cues for Phenological Events in the Flowering Cycle in Avocado.Plants (Basel). 2023 Jun 13;12(12):2304. doi: 10.3390/plants12122304. Plants (Basel). 2023. PMID: 37375929 Free PMC article.
-
Persian Walnut (Juglans regia L.) Bud Dormancy Dynamics in Northern Patagonia, Argentina.Front Plant Sci. 2022 Feb 3;12:803878. doi: 10.3389/fpls.2021.803878. eCollection 2021. Front Plant Sci. 2022. PMID: 35185955 Free PMC article.
-
Small RNA and Degradome Sequencing in Floral Bud Reveal Roles of miRNAs in Dormancy Release of Chimonanthus praecox.Int J Mol Sci. 2023 Feb 20;24(4):4210. doi: 10.3390/ijms24044210. Int J Mol Sci. 2023. PMID: 36835618 Free PMC article.
References
-
- Lang GA, Early JD, Darnell RL, Martin GC. Endo-, para-, and eco-dormancy: physiological terminology and classification for dormancy research. Hort. science. 1987;22:371–377.
-
- Samish MR. Dormancy in woody plants. Annu. Rev. Plant Physiol. 1954;5:183–204. doi: 10.1146/annurev.pp.05.060154.001151. - DOI
-
- Arora R, Rowland LJ, Tanino K. Induction and release of bud dormancy in woody perennials: a science comes of age. Hort. Sci. 2003;38:911–921.
-
- Howe GT, et al. Physiological and genetic approaches to studying endodormancy-related traits in Populus. Hort. science. 1999;34:1174b–1184.

