Is the oral microbiome a source to enhance mucosal immunity against infectious diseases?
- PMID: 34078913
- PMCID: PMC8172910
- DOI: 10.1038/s41541-021-00341-4
Is the oral microbiome a source to enhance mucosal immunity against infectious diseases?
Abstract
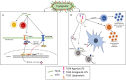
Mucosal tissues act as a barrier throughout the oral, nasopharyngeal, lung, and intestinal systems, offering first-line protection against potential pathogens. Conventionally, vaccines are applied parenterally to induce serotype-dependent humoral response but fail to drive adequate mucosal immune protection for viral infections such as influenza, HIV, and coronaviruses. Oral mucosa, however, provides a vast immune repertoire against specific microbial pathogens and yet is shaped by an ever-present microbiome community that has co-evolved with the host over thousands of years. Adjuvants targeting mucosal T-cells abundant in oral tissues can promote soluble-IgA (sIgA)-specific protection to confer increased vaccine efficacy. Th17 cells, for example, are at the center of cell-mediated immunity and evidence demonstrates that protection against heterologous pathogen serotypes is achieved with components from the oral microbiome. At the point of entry where pathogens are first encountered, typically the oral or nasal cavity, the mucosal surfaces are layered with bacterial cohabitants that continually shape the host immune profile. Constituents of the oral microbiome including their lipids, outer membrane vesicles, and specific proteins, have been found to modulate the Th17 response in the oral mucosa, playing important roles in vaccine and adjuvant designs. Currently, there are no approved adjuvants for the induction of Th17 protection, and it is critical that this research is included in the preparedness for the current and future pandemics. Here, we discuss the potential of oral commensals, and molecules derived thereof, to induce Th17 activity and provide safer and more predictable options in adjuvant engineering to prevent emerging infectious diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Mubarak A, et al. A dynamic relationship between mucosal T helper type 17 and regulatory T-cell populations in nasopharynx evolves with age and associates with the clearance of pneumococcal carriage in humans. Clin. Microbiol. Infect. 2016;22:736.e1–736.e7. doi: 10.1016/j.cmi.2016.05.017. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

