Lipid profile of bovine grade-1 blastocysts produced either in vivo or in vitro before and after slow freezing process
- PMID: 34078963
- PMCID: PMC8172931
- DOI: 10.1038/s41598-021-90870-8
Lipid profile of bovine grade-1 blastocysts produced either in vivo or in vitro before and after slow freezing process
Abstract
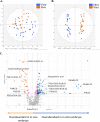
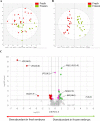
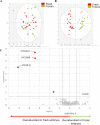
Currently, in vitro embryo production (IVP) is successfully commercially applied in cattle. However, the high sensitivity of embryos to cryopreservation in comparison to in vivo (IVD) embryos slows the dissemination of this biotechnology. Reduced cryotolerance is frequently associated with lipid accumulation in the cytoplasm mainly due to in vitro culture conditions. The objective of this study was to evaluate the lipid composition of biopsied and sexed embryos, produced either in vivo or in vitro from the same Holstein heifers before and after a slow freezing protocol. Lipid extracts were analysed by liquid chromatography-high resolution mass spectrometry, which enabled the detection of 496 features. Our results highlighted a lipid enrichment of IVP embryos in triglycerides and oxidised glycerophospholipids and a reduced abundance in glycerophospholipids. The slow freezing process affected the lipid profiles of IVP and IVD embryos similarly. Lysophosphatidylcholine content was reduced when embryos were frozen/thawed. In conclusion, the embryonic lipid profile is impacted by IVP and slow freezing protocols but not by sex. Lysophosphatidylcholine seemed highly sensitive to cryopreservation and might contribute to explain the lower quality of frozen embryos. Further studies are required to improve embryo freezability by modulating the lipidome.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Novel Synthetic oviductal fluid for Conventional Freezing 1 (SCF1) culture medium improves development and cryotolerance of in vitro produced Holstein embryos.J Anim Sci. 2022 Mar 1;100(3):skac043. doi: 10.1093/jas/skac043. J Anim Sci. 2022. PMID: 35148394 Free PMC article.
-
Effects of the donor factors and freezing protocols on the bovine embryonic lipid profile†.Biol Reprod. 2022 Mar 19;106(3):597-612. doi: 10.1093/biolre/ioab198. Biol Reprod. 2022. PMID: 34718415 Free PMC article.
-
Phosphatidylcholine and sphingomyelin profiles vary in Bos taurus indicus and Bos taurus taurus in vitro- and in vivo-produced blastocysts.Biol Reprod. 2012 Dec 6;87(6):130. doi: 10.1095/biolreprod.112.102897. Print 2012 Jun. Biol Reprod. 2012. PMID: 23053436
-
Recent progress in bovine in vitro-derived embryo cryotolerance: Impact of in vitro culture systems, advances in cryopreservation and future considerations.Reprod Domest Anim. 2020 Jun;55(6):659-676. doi: 10.1111/rda.13667. Epub 2020 Apr 17. Reprod Domest Anim. 2020. PMID: 32144939 Review.
-
Cryopreservation and sexing of in vivo- and in vitro-produced bovine embryos for their practical use.J Reprod Dev. 2004 Feb;50(1):29-38. doi: 10.1262/jrd.50.29. J Reprod Dev. 2004. PMID: 15007199 Review.
Cited by
-
The recipient metabolome explains the asymmetric ovarian impact on fetal sex development after embryo transfer in cattle.J Anim Sci. 2024 Jan 3;102:skae081. doi: 10.1093/jas/skae081. J Anim Sci. 2024. PMID: 38567815 Free PMC article.
-
Supplementation of Forskolin and Linoleic Acid During IVC Improved the Developmental and Vitrification Efficiency of Bovine Embryos.Int J Mol Sci. 2025 Apr 27;26(9):4151. doi: 10.3390/ijms26094151. Int J Mol Sci. 2025. PMID: 40362390 Free PMC article.
-
Impact of antral follicle count on follicular-luteal characteristics, superovulatory response, and embryo quality in Sahiwal cows.Front Vet Sci. 2024 Oct 22;11:1494065. doi: 10.3389/fvets.2024.1494065. eCollection 2024. Front Vet Sci. 2024. PMID: 39502948 Free PMC article.
-
The Metabolic Signature of In Vitro Produced Bovine Embryos Helps Predict Pregnancy and Birth after Embryo Transfer.Metabolites. 2021 Jul 27;11(8):484. doi: 10.3390/metabo11080484. Metabolites. 2021. PMID: 34436426 Free PMC article.
-
The proteomic analysis of bovine embryos developed in vivo or in vitro reveals the contribution of the maternal environment to early embryo.BMC Genomics. 2022 Dec 19;23(1):839. doi: 10.1186/s12864-022-09076-5. BMC Genomics. 2022. PMID: 36536309 Free PMC article.
References
-
- Hasler JF, et al. Production, freezing and transfer of bovine IVF embryos and subsequent calving results. Theriogenology. 1995;43:141–152. doi: 10.1016/0093-691X(94)00020-U. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

