Aberrant activity of mitochondrial NCLX is linked to impaired synaptic transmission and is associated with mental retardation
- PMID: 34079053
- PMCID: PMC8172942
- DOI: 10.1038/s42003-021-02114-0
Aberrant activity of mitochondrial NCLX is linked to impaired synaptic transmission and is associated with mental retardation
Erratum in
-
Author Correction: Aberrant activity of mitochondrial NCLX is linked to impaired synaptic transmission and is associated with mental retardation.Commun Biol. 2021 Jun 14;4(1):755. doi: 10.1038/s42003-021-02312-w. Commun Biol. 2021. PMID: 34127779 Free PMC article. No abstract available.
Abstract
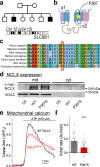
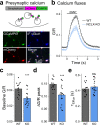
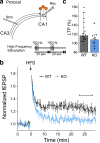
Calcium dynamics control synaptic transmission. Calcium triggers synaptic vesicle fusion, determines release probability, modulates vesicle recycling, participates in long-term plasticity and regulates cellular metabolism. Mitochondria, the main source of cellular energy, serve as calcium signaling hubs. Mitochondrial calcium transients are primarily determined by the balance between calcium influx, mediated by the mitochondrial calcium uniporter (MCU), and calcium efflux through the sodium/lithium/calcium exchanger (NCLX). We identified a human recessive missense SLC8B1 variant that impairs NCLX activity and is associated with severe mental retardation. On this basis, we examined the effect of deleting NCLX in mice on mitochondrial and synaptic calcium homeostasis, synaptic activity, and plasticity. Neuronal mitochondria exhibited basal calcium overload, membrane depolarization, and a reduction in the amplitude and rate of calcium influx and efflux. We observed smaller cytoplasmic calcium transients in the presynaptic terminals of NCLX-KO neurons, leading to a lower probability of release and weaker transmission. In agreement, synaptic facilitation in NCLX-KO hippocampal slices was enhanced. Importantly, deletion of NCLX abolished long term potentiation of Schaffer collateral synapses. Our results show that NCLX controls presynaptic calcium transients that are crucial for defining synaptic strength as well as short- and long-term plasticity, key elements of learning and memory processes.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

