Nivolumab-Induced Alopecia Areata: A Case Report and Literature Review
- PMID: 34079191
- PMCID: PMC8137334
- DOI: 10.5021/ad.2021.33.3.284
Nivolumab-Induced Alopecia Areata: A Case Report and Literature Review
Abstract
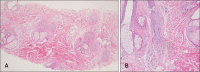
Nivolumab (anti-PD-1) currently used in many cancers. With the usage of nivolumab increased, many cutaneous side effects were reported including maculopapular rash, lichenoid reactions, vitiligo, bullous disorders, psoriasis exacerbation, and alopecia areata (AA). Here, we report AA after nivolumab for treatment of hepatocellular carcinomas (HCC). A 55-year-old male presented with multiple hairless patch from 1 month ago. He suffered HCC and treated with nivolumab for 6 months after hepatectomy. He treated for hair loss with triamcinolone intra-lesional injection without improvement. We performed skin biopsy on the scalp. Histopathologic findings revealed decreased of hair follicles on the horizontal section with lymphocyte infiltration on the perifollicular area on the vertical section. Clinicopathologic findings were agreed with AA. Considering lack of previous history of AA and hairless patches with 6 months after nivolumab injection, we diagnosed him as nivolumab induced AA. Treatment included topical steroid, and minoxidil. No regrowth of hair was noted after 4 months of follow-up. Nivolimumab induced AA is rare side effect. Pathogenesis of nivolumab induced AA remain unclear. But our case is likely related to nivolumab, known to induce immune related adverse events, and given in the delay of a few months between introduction and the occurrence of the hair loss. Here, we reports nivolmumab induced AA; rare side effect.
Keywords: Alopecia areata; Nivolumab.
Copyright © 2021 The Korean Dermatological Association and The Korean Society for Investigative Dermatology.
Conflict of interest statement
CONFLICTS OF INTEREST: The authors have nothing to disclose.
Figures


References
-
- Azoury SC, Straughan DM, Shukla V. Immune checkpoint inhibitors for cancer therapy: clinical efficacy and safety. Curr Cancer Drug Targets. 2015;15:452–462. - PubMed
-
- Tsirigotis P, Savani BN, Nagler A. Programmed death-1 immune checkpoint blockade in the treatment of hematological malignancies. Ann Med. 2016;48:428–439. - PubMed
-
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345–361. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

