A Randomized, Controlled, Prospective Study of the Effectiveness and Safety of an Intracanalicular Dexamethasone Ophthalmic Insert (0.4 Mg) for the Treatment of Post-Operative Inflammation in Patients Undergoing Refractive Lens Exchange (RLE)
- PMID: 34079218
- PMCID: PMC8166314
- DOI: 10.2147/OPTH.S311070
A Randomized, Controlled, Prospective Study of the Effectiveness and Safety of an Intracanalicular Dexamethasone Ophthalmic Insert (0.4 Mg) for the Treatment of Post-Operative Inflammation in Patients Undergoing Refractive Lens Exchange (RLE)
Abstract
Purpose: To determine patient preference and treatment outcomes with an intracanalicular dexamethasone (0.4 mg) insert compared to standard steroid drop regimen in the contralateral eye following bilateral RLE surgery.
Methods: This is a prospective, open-label, interventional, randomized, controlled study in 20 subjects who underwent bilateral RLE. Each patient served as their own control with one eye randomized to the intracanalicular insert (Group A) placed at the time of surgery and the contralateral randomized to topical corticosteroid drops (Group B). All eyes received intracameral moxifloxacin at the time of surgery, and post-operatively, topical moxifloxacin QID for one week and topical NSAID daily for four weeks. Post-operative evaluations were performed on Day 1, Week 1, and Week 4-8.
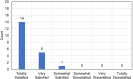
Results: Twenty patients participated. At 4-8 weeks post-operation, 90% of patients evaluated with the COMTOL questionnaire preferred the intracanalicular insert while 10% preferred the topical steroid. Comparative analysis using the visual analog scale showed no difference in pain between the study and control group. No statistical difference was shown in post-operative corneal staining, anterior chamber cell count, anterior chamber flare or intraocular pressure. Mean LogMAR UCVA at 4-8 weeks post-operation was 0.06 (± 0.230) in the study group and 0.065 (± 0.241) in the control group, which was not statistically or clinically different (p > 0.05).
Conclusion: Patients undergoing bilateral RLE expressed a strong preference towards the use of an intracanalicular insert over a topical steroid for post-operative steroid treatment. There was no clinically or statistically significant difference in outcomes, including rate of cystoid macular edema, visual acuity and elevation of intraocular pressure.
National clinical trial number: 04549935.
Keywords: cystoid macular edema; patient preference; punctal plug; steroid; visual acuity.
© 2021 Larsen et al.
Conflict of interest statement
The authors report no conflicts of interest for this work.
Figures



Similar articles
-
Prospective, Randomized, Fellow Eye-Controlled Study of Postoperative Pain and Inflammation Control with an Intracanalicular Dexamethasone 0.4 mg Ophthalmic Insert Following Small Incision Lenticule Extraction.Clin Ophthalmol. 2022 Nov 22;16:3895-3904. doi: 10.2147/OPTH.S390815. eCollection 2022. Clin Ophthalmol. 2022. PMID: 36444207 Free PMC article.
-
A Randomized, Prospective, Observer-Masked Study Comparing Dropless Treatment Regimen Using Intracanalicular Dexamethasone Insert, Intracameral Ketorolac, and Intracameral Moxifloxacin versus Conventional Topical Therapy to Control Postoperative Pain and Inflammation in Cataract Surgery.Clin Ophthalmol. 2023 Aug 15;17:2349-2356. doi: 10.2147/OPTH.S422502. eCollection 2023. Clin Ophthalmol. 2023. PMID: 37600148 Free PMC article. Clinical Trial.
-
The Effectiveness and Safety of Dextenza 0.4 mg for the Treatment of Postoperative Inflammation and Pain in Patients After Photorefractive Keratectomy: The RESTORE Trial.J Refract Surg. 2021 Sep;37(9):590-594. doi: 10.3928/1081597X-20210610-05. Epub 2021 Sep 1. J Refract Surg. 2021. PMID: 34506241 Clinical Trial.
-
Dexamethasone Intracanalicular Insert: A Review in Treating Post-Surgical Ocular Pain and Inflammation.Drugs. 2020 Jul;80(11):1101-1108. doi: 10.1007/s40265-020-01344-6. Drugs. 2020. PMID: 32588339 Free PMC article. Review.
-
Dexamethasone 0.4mg Sustained-Release Intracanalicular Insert in the Management of Ocular Inflammation and Pain Following Ophthalmic Surgery: Design, Development and Place in Therapy.Clin Ophthalmol. 2020 Jan 13;14:89-94. doi: 10.2147/OPTH.S238756. eCollection 2020. Clin Ophthalmol. 2020. PMID: 32021072 Free PMC article. Review.
Cited by
-
Prospective, Randomized, Fellow Eye-Controlled Study of Postoperative Pain and Inflammation Control with an Intracanalicular Dexamethasone 0.4 mg Ophthalmic Insert Following Small Incision Lenticule Extraction.Clin Ophthalmol. 2022 Nov 22;16:3895-3904. doi: 10.2147/OPTH.S390815. eCollection 2022. Clin Ophthalmol. 2022. PMID: 36444207 Free PMC article.
-
A Randomized, Prospective, Observer-Masked Study Comparing Dropless Treatment Regimen Using Intracanalicular Dexamethasone Insert, Intracameral Ketorolac, and Intracameral Moxifloxacin versus Conventional Topical Therapy to Control Postoperative Pain and Inflammation in Cataract Surgery.Clin Ophthalmol. 2023 Aug 15;17:2349-2356. doi: 10.2147/OPTH.S422502. eCollection 2023. Clin Ophthalmol. 2023. PMID: 37600148 Free PMC article. Clinical Trial.
-
Early Real-World Physician Experience with an Intracanalicular Dexamethasone Insert.Clin Ophthalmol. 2022 Aug 6;16:2429-2440. doi: 10.2147/OPTH.S372440. eCollection 2022. Clin Ophthalmol. 2022. PMID: 35968052 Free PMC article.
-
Thermal Pulsation with or without Dexamethasone Intracanalicular Insert for Meibomian Gland Dysfunction: A Prospective, Masked Trial.Clin Ophthalmol. 2022 May 12;16:1477-1485. doi: 10.2147/OPTH.S359719. eCollection 2022. Clin Ophthalmol. 2022. PMID: 35585875 Free PMC article.
-
DEXTENZA versus Topical Steroid or Antihistamine Therapy for Treatment of Allergic Conjunctivitis.Clin Ophthalmol. 2024 Feb 14;18:473-480. doi: 10.2147/OPTH.S440840. eCollection 2024. Clin Ophthalmol. 2024. PMID: 38375441 Free PMC article. Clinical Trial.
References
-
- Walters T, Bafna S, Vold S, et al. Efficacy and safety of sustained release dexamethasone for the treatment of ocular pain and inflammation after cataract surgery: results from two phase 3 studies. J Clin Exp Ophthalmol. 2016;7:1–11. doi:10.4172/2155-9570.1000572 - DOI
-
- Tyson SL, Bafna S, Gira JP, et al. Multicenter randomized phase 3 study of a sustained release intracanalicular dexamethasone insert for treatment of ocular inflammation and pain after cataract surgery. [published correction appears in J Cataract Refract Surg. 2019;45(6):895]. J Cataract Refract Surg. 2019;45(2):204–212. doi:10.1016/j.jcrs.2018.09.023 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

