Electronic Monitoring Feedback for Improving Medication Adherence and Clinical Outcomes in Early Rheumatoid Arthritis: A Randomized Clinical Trial
- PMID: 34079231
- PMCID: PMC8164714
- DOI: 10.2147/PPA.S297170
Electronic Monitoring Feedback for Improving Medication Adherence and Clinical Outcomes in Early Rheumatoid Arthritis: A Randomized Clinical Trial
Abstract
Background: Non-adherence to medication (range 30-107%) is a major issue in patients with rheumatoid arthritis (RA). Previous research has shown that electronic monitoring feedback (EMF) might be an effective strategy to improve medication adherence in chronic conditions. Therefore, this study investigated the effectiveness of electronic monitoring feedback in patients with early RA to improve medication adherence and clinical outcomes compared to usual care.
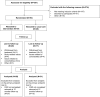
Methods: An open-label randomized clinical trial was performed to compare EMF with standard care during a 12-month follow-up period on two sites of the Sint Maartenskliniek (Nijmegen and Boxmeer) in the Netherlands. Patients were eligible if they: (1) had a (working) diagnosis of early RA, (2) were currently using methotrexate, (3) were aged ≥18 years, and (4) had a life expectancy of ≥12 months. Primary outcome was the difference in proportion of non-adherent patients measured with the Compliance Questionnaire on Rheumatology after 12 months. Secondary outcomes were beliefs about medicines, medication adherence measured with the MMAS-8®, patients' health status, prescription of biologic DMARDs, and disease activity after 12 months.
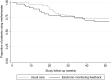
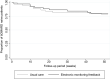
Results: Of the 367 initially-invited patients, 93 patients with early RA agreed to participate in this study. No significant difference was found in the proportion of non-adherent patients between the intervention arm and the usual care arm after 12 months follow-up (60.0% and 61.3%, p=0.93, respectively). Patients in the intervention arm tended to discontinue methotrexate earlier than patients in the usual care arm (median time in weeks: 15.7 (9.1-33.6) and 21.9 (19-28.4), respectively, p=0.31), whereas patients in the usual care arm tended to initiate biologic DMARDs earlier than those in the intervention arm (median time in weeks: 11.9 (5.7-22) and 17 (9.9-40.9), respectively, p=0.55).
Conclusion: This study illustrates the challenge of targeting non-adherence with EMF in patients with early RA and shares important lessons learned about designing adherence intervention trials with respect to study attrition, accounting for drug survival, intervention fidelity, intervention uptake, and technical aspects.
Keywords: DMARDs; clinical outcomes; disease activity; medication adherence; randomized clinical trial; rheumatoid arthritis.
© 2021 van Heuckelum et al.
Conflict of interest statement
Dr Milou van Heuckelum reports receipt of grants from the Dutch Arthritis Foundation during the conduct of the study and grants from the Dutch Arthritis Foundation outside the submitted work. The authors reported no other potential conflicts of interest in this work.
Figures

Similar articles
-
Gaming for Adherence to Medication using Ehealth in Rheumatoid arthritis (GAMER) study: a randomised controlled trial.RMD Open. 2022 Nov;8(2):e002616. doi: 10.1136/rmdopen-2022-002616. RMD Open. 2022. PMID: 36410776 Free PMC article. Clinical Trial.
-
Effectiveness of a group-based intervention to change medication beliefs and improve medication adherence in patients with rheumatoid arthritis: a randomized controlled trial.Patient Educ Couns. 2014 Mar;94(3):356-61. doi: 10.1016/j.pec.2013.12.002. Epub 2013 Dec 11. Patient Educ Couns. 2014. PMID: 24388126 Clinical Trial.
-
Examining Time to Initiation of Biologic Disease-modifying Antirheumatic Drugs and Medication Adherence and Persistence Among Texas Medicaid Recipients With Rheumatoid Arthritis.Clin Ther. 2016 Mar;38(3):646-54. doi: 10.1016/j.clinthera.2016.01.022. Epub 2016 Feb 18. Clin Ther. 2016. PMID: 26899313
-
Psychological interventions for improving adherence to inhaled therapies in people with cystic fibrosis.Cochrane Database Syst Rev. 2023 Mar 29;3(3):CD013766. doi: 10.1002/14651858.CD013766.pub2. Cochrane Database Syst Rev. 2023. PMID: 36989170 Free PMC article. Review.
-
Down-titration and discontinuation strategies of tumour necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity.Cochrane Database Syst Rev. 2019 May 24;5(5):CD010455. doi: 10.1002/14651858.CD010455.pub3. Cochrane Database Syst Rev. 2019. PMID: 31125448 Free PMC article.
Cited by
-
Gaming for Adherence to Medication using Ehealth in Rheumatoid arthritis (GAMER) study: a randomised controlled trial.RMD Open. 2022 Nov;8(2):e002616. doi: 10.1136/rmdopen-2022-002616. RMD Open. 2022. PMID: 36410776 Free PMC article. Clinical Trial.
-
The Effect of Self-Administration of Medication During Hospitalization on Patient's Self-Efficacy and Medication Adherence After Discharge.Patient Prefer Adherence. 2022 Sep 28;16:2683-2693. doi: 10.2147/PPA.S375295. eCollection 2022. Patient Prefer Adherence. 2022. PMID: 36196066 Free PMC article.
-
Drug-related problems reported by patients with rheumatic diseases: an observational study.BMC Rheumatol. 2023 Apr 18;7(1):7. doi: 10.1186/s41927-023-00326-x. BMC Rheumatol. 2023. PMID: 37069634 Free PMC article.
-
What Do We Know about Medication Adherence Interventions in Inflammatory Bowel Disease, Multiple Sclerosis and Rheumatoid Arthritis? A Scoping Review of Randomised Controlled Trials.Patient Prefer Adherence. 2023 Dec 13;17:3265-3303. doi: 10.2147/PPA.S424024. eCollection 2023. Patient Prefer Adherence. 2023. PMID: 38111690 Free PMC article.
References
LinkOut - more resources
Full Text Sources

