Current Opinion on the use of Direct Oral Anticoagulants for the Prophylaxis of Venous Thromboembolism among Medical Inpatients
- PMID: 34079269
- PMCID: PMC8165214
- DOI: 10.2147/TCRM.S271439
Current Opinion on the use of Direct Oral Anticoagulants for the Prophylaxis of Venous Thromboembolism among Medical Inpatients
Abstract
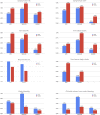
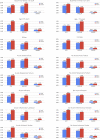
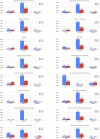
Venous thromboembolism (VTE) is a known cause of morbidity and mortality, especially among acutely ill medical patients. Although VTE prophylaxis is part of post-discharge clinical care in surgical patients, there is controversy regarding its use in acutely ill medical patients and the current guideline statements suggest against its routine use. Recent clinical trials (APEX, MAGELLAN and MARINER) compared the safety and efficacy of direct oral anticoagulants (including betrixaban and rivaroxaban) with the standard of the care, enoxaparin, to identify the risk-benefit tradeoff. In this review, we summarized the key findings from these trials and substudies and recent updates in society guidelines regarding VTE prevention. In addition, we discussed the potential barriers, cost-effectiveness, and COVID-19 with respect to the implementation of extended-duration or post-discharge usage of direct oral anticoagulants.
Keywords: betrixaban; enoxaparin; major bleeding; medically ill; rivaroxaban; thromboembolic events.
© 2021 Lee et al.
Conflict of interest statement
The authors reported no conflicts of interest for this work.
Figures
Similar articles
-
Extended thromboprophylaxis in the acutely ill medical patient after hospitalization - a paradigm shift in post-discharge thromboprophylaxis.Hosp Pract (1995). 2018 Feb;46(1):5-15. doi: 10.1080/21548331.2018.1410053. Epub 2017 Nov 30. Hosp Pract (1995). 2018. PMID: 29171776 Review.
-
Betrixaban for first-line venous thromboembolism prevention in acute medically ill patients with risk factors for venous thromboembolism.Expert Rev Cardiovasc Ther. 2018 Nov;16(11):845-855. doi: 10.1080/14779072.2018.1534068. Epub 2018 Oct 15. Expert Rev Cardiovasc Ther. 2018. PMID: 30296387 Review.
-
Extended Venous Thromboembolism Prophylaxis in Medically Ill Patients: An NATF Anticoagulation Action Initiative.Am J Med. 2020 May;133 Suppl 1:1-27. doi: 10.1016/j.amjmed.2019.12.001. Am J Med. 2020. PMID: 32362349
-
Extended-duration betrixaban versus shorter-duration enoxaparin for venous thromboembolism prophylaxis in critically ill medical patients: an APEX trial substudy.Intensive Care Med. 2019 Apr;45(4):477-487. doi: 10.1007/s00134-019-05565-6. Epub 2019 Feb 18. Intensive Care Med. 2019. PMID: 30778649 Clinical Trial.
-
Betrixaban: A New Oral Factor Xa Inhibitor for Extended Venous Thromboembolism Prophylaxis in High-Risk Hospitalized Patients.Ann Pharmacother. 2018 Jun;52(6):554-561. doi: 10.1177/1060028018754383. Epub 2018 Jan 17. Ann Pharmacother. 2018. PMID: 29338293
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous