Targeting Airway Smooth Muscle Hypertrophy in Asthma: An Approach Whose Time Has Come
- PMID: 34079293
- PMCID: PMC8164696
- DOI: 10.2147/JAA.S280247
Targeting Airway Smooth Muscle Hypertrophy in Asthma: An Approach Whose Time Has Come
Abstract
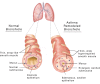
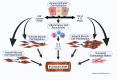
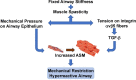
Airway smooth muscle (ASM) cell dysfunction is an important component of several obstructive pulmonary diseases, particularly asthma. External stimuli such as allergens, dust, air pollutants, and change in environmental temperatures provoke ASM cell hypertrophy, proliferation, and migration without adequate mechanistic controls. ASM cells can switch between quiescent, migratory, and proliferative phenotypes in response to extracellular matrix proteins, growth factors, and other soluble mediators. While some aspects of airway hypertrophy and remodeling could have beneficial effects, in many cases these contribute to a clinical phenotype of difficult to control asthma. In this review, we discuss the factors responsible for ASM hypertrophy and proliferation in asthma, focusing on cytokines, growth factors, and ion transporters, and discuss existing and potential approaches that specifically target ASM hypertrophy to reduce the ASM mass and improve asthma symptoms. The goal of this review is to highlight strategies that appear ready for translational investigations to improve asthma therapy.
Keywords: airway remodeling; airway smooth muscle cells; hypertrophy; proliferation.
© 2021 Chetty and Nielsen.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Ebina M, Yaegashi H, Takahashi T, Motomiya M, Tanemura M. Distribution of smooth muscles along the bronchial tree. A morphometric study of ordinary autopsy lungs. Am Rev Respir Dis. 1990;141(5 Pt 1):1322–1326. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

