Comparative Cost and Effectiveness of a New Algorithm for Early Lyme Disease Diagnosis: Evaluation in US, Germany, and Italy
- PMID: 34079307
- PMCID: PMC8165099
- DOI: 10.2147/CEOR.S306391
Comparative Cost and Effectiveness of a New Algorithm for Early Lyme Disease Diagnosis: Evaluation in US, Germany, and Italy
Abstract
Purpose: This Lyme disease early detection economic model, for patients with suspected Lyme disease without erythema migrans (EM), compares outcomes of standard two-tier testing (sTTT), modified two-tier testing (mTTT) and the DiaSorin Lyme Detection Algorithm (LDA), a combination of both serology tests and Interferon-ɤ Release Assay.
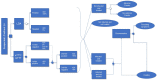
Patients and methods: A patient-level simulation model was built to incorporate effectiveness estimation from a structured focused literature review, and health-care cost inputs for the United States, Germany, and Italy. Simulated clinical outcomes were 1) percent of patients with timely and correct diagnosis, 2) patients appropriately treated and exposed to antibiotics therapy, and 3) patients with late Lyme disease manifestations. Expected health outcomes were expressed in terms of differences in quality-adjusted life years (QALYs) due to disseminated Lyme disease and persisting symptoms, and economic outcomes were analyzed from a third-party payer perspective.
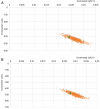
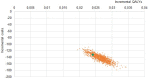
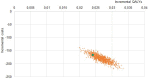
Results: The DiaSorin LDA resulted in a better sensitivity compared to sTTT and mTTT, 84% vs 49% and 45%, respectively, in the base case (13% of infected patients in the tested population). Due to the improved diagnostic performance, the LDA-based strategy is expected to be more effective, providing mean incremental 0.024 QALYs per tested patient, or 0.19 per infected patient. Furthermore, from a third-party payer perspective, the adoption of the LDA-based strategy would reduce the expected health-care cost for suspected and confirmed Lyme disease by roughly 40%, ie about $410, €130, and €170 per tested patient in the United States, Germany, and Italy, respectively, compared to sTTT. The results are most sensitive to the infection rate in the tested population, with LDA maintaining a cost advantage for Lyme disease active infection rates ≥0.8-2.5%.
Conclusion: LDA early diagnostic testing and subsequent treatment of subjects with early Lyme disease without EM are expected to outperform traditional management strategies both clinically and economically in the US, Germany, and Italy.
Keywords: IGRA; Lyme borreliosis; QALYs; early diagnostics; health-care cost; serology.
© 2021 Pradelli et al.
Conflict of interest statement
We acknowledge the full financial support of DiaSorin SpA for this research manuscript. DiaSorin provided support in the form of salaries for authors Fabrizio Bonelli, Mariella Calleri, Matteo Pinciroli, Beatrice Grassi, and Hirad Houshmand. Lorenzo Pradelli is the co-owner and an employee of AdRes and has received project funding from DiaSorin SpA. Dr Maurizio Ruscio has received consulting honoraria from DiaSorin SpA.
Figures


References
-
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089–1134. - PubMed
LinkOut - more resources
Full Text Sources

