Methylene Blue Application to Lessen Pain: Its Analgesic Effect and Mechanism
- PMID: 34079436
- PMCID: PMC8165385
- DOI: 10.3389/fnins.2021.663650
Methylene Blue Application to Lessen Pain: Its Analgesic Effect and Mechanism
Abstract
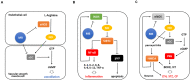
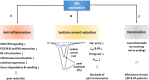
Methylene blue (MB) is a cationic thiazine dye, widely used as a biological stain and chemical indicator. Growing evidence have revealed that MB functions to restore abnormal vasodilation and notably it is implicated even in pain relief. Physicians began to inject MB into degenerated disks to relieve pain in patients with chronic discogenic low back pain (CDLBP), and some of them achieved remarkable outcomes. For osteoarthritis and colitis, MB abates inflammation by suppressing nitric oxide production, and ultimately relieves pain. However, despite this clinical efficacy, MB has not attracted much public attention in terms of pain relief. Accordingly, this review focuses on how MB lessens pain, noting three major actions of this dye: anti-inflammation, sodium current reduction, and denervation. Moreover, we showed controversies over the efficacy of MB on CDLBP and raised also toxicity issues to look into the limitation of MB application. This analysis is the first attempt to illustrate its analgesic effects, which may offer a novel insight into MB as a pain-relief dye.
Keywords: anti-inflammation; denervation; methylene blue; pain; sodium current.
Copyright © 2021 Lee and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Methylene blue induces an analgesic effect by significantly decreasing neural firing rates and improves pain behaviors in rats.Biochem Biophys Res Commun. 2021 Feb 19;541:36-42. doi: 10.1016/j.bbrc.2021.01.008. Epub 2021 Jan 16. Biochem Biophys Res Commun. 2021. PMID: 33465740
-
Methylene blue in the treatment of discogenic low back pain.Pain Physician. 2012 Jul-Aug;15(4):333-8. Pain Physician. 2012. PMID: 22828687
-
A randomized placebo-controlled trial of intradiscal methylene blue injection for the treatment of chronic discogenic low back pain.Pain. 2010 Apr;149(1):124-129. doi: 10.1016/j.pain.2010.01.021. Epub 2010 Feb 18. Pain. 2010. PMID: 20167430 Clinical Trial.
-
Intradiscal Methylene Blue Injection for Discogenic Low Back Pain: A Meta-Analysis.Pain Pract. 2019 Jan;19(1):118-129. doi: 10.1111/papr.12725. Epub 2018 Aug 30. Pain Pract. 2019. PMID: 30039642 Review.
-
Intradiskal Injection of Methylene Blue for Discogenic Back Pain: A Meta-Analysis of Randomized Controlled Trials.J Neurol Surg A Cent Eur Neurosurg. 2021 Mar;82(2):161-165. doi: 10.1055/s-0040-1721015. Epub 2021 Jan 21. J Neurol Surg A Cent Eur Neurosurg. 2021. PMID: 33477188 Review.
Cited by
-
Analgesic effect of ropivacaine combined with methylene blue in fascia Iliaca block for patients undergoing hip arthroplasty.BMC Musculoskelet Disord. 2025 Mar 14;26(1):256. doi: 10.1186/s12891-025-08490-6. BMC Musculoskelet Disord. 2025. PMID: 40087701 Free PMC article. Clinical Trial.
-
Increased Expression of Inflammatory Cytokines and Discogenic Neck Pain.Orthop Surg. 2024 Jan;16(1):227-233. doi: 10.1111/os.13963. Epub 2023 Dec 14. Orthop Surg. 2024. PMID: 38097400 Free PMC article.
-
Methylene Blue for the Treatment of Radiation-Induced Oral Mucositis during Head and Neck Cancer Treatment: An Uncontrolled Cohort.Cancers (Basel). 2023 Aug 7;15(15):3994. doi: 10.3390/cancers15153994. Cancers (Basel). 2023. PMID: 37568810 Free PMC article.
-
Redispersible CuO nanoparticles: preparation and photocatalytic capacity for the degradation of methylene blue.RSC Adv. 2025 Jun 5;15(24):19023-19033. doi: 10.1039/d5ra02244d. eCollection 2025 Jun 4. RSC Adv. 2025. PMID: 40476236 Free PMC article.
-
Addressing Pain in Oral Mucositis: Narrative Review of Current Practices and Emerging Treatments.J Pain Res. 2025 Jul 23;18:3723-3741. doi: 10.2147/JPR.S533351. eCollection 2025. J Pain Res. 2025. PMID: 40718715 Free PMC article. Review.
References
-
- Bach K. K., Lindsay F. W., Berg L. S., Howard R. S. (2004). Prolonged postoperative disorientation after methylene blue infusion during parathyroidectomy. Anesth Analg 99 1573–1574. 10.1213/01.ane.0000134860.73875.cf - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

