Molecular Pathobiology of the Cerebrovasculature in Aging and in Alzheimers Disease Cases With Cerebral Amyloid Angiopathy
- PMID: 34079449
- PMCID: PMC8166206
- DOI: 10.3389/fnagi.2021.658605
Molecular Pathobiology of the Cerebrovasculature in Aging and in Alzheimers Disease Cases With Cerebral Amyloid Angiopathy
Abstract
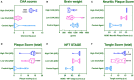
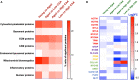
Cerebrovascular dysfunction and cerebral amyloid angiopathy (CAA) are hallmark features of Alzheimer's disease (AD). Molecular damage to cerebrovessels in AD may result in alterations in vascular clearance mechanisms leading to amyloid deposition around blood vessels and diminished neurovascular-coupling. The sequelae of molecular events leading to these early pathogenic changes remains elusive. To address this, we conducted a comprehensive in-depth molecular characterization of the proteomic changes in enriched cerebrovessel fractions isolated from the inferior frontal gyrus of autopsy AD cases with low (85.5 ± 2.9 yrs) vs. high (81 ± 4.4 yrs) CAA score, aged-matched control (87.4 ± 1.5 yrs) and young healthy control (47 ± 3.3 yrs) cases. We employed a 10-plex tandem isobaric mass tag approach in combination with our ultra-high pressure liquid chromatography MS/MS (Q-Exactive) method. Enriched cerebrovascular fractions showed very high expression levels of proteins specific to endothelial cells, mural cells (pericytes and smooth muscle cells), and astrocytes. We observed 150 significantly regulated proteins in young vs. aged control cerebrovessels. The top pathways significantly modulated with aging included chemokine, reelin, HIF1α and synaptogenesis signaling pathways. There were 213 proteins significantly regulated in aged-matched control vs. high CAA cerebrovessels. The top three pathways significantly altered from this comparison were oxidative phosphorylation, Sirtuin signaling pathway and TCA cycle II. Comparison between low vs. high CAA cerebrovessels identified 84 significantly regulated proteins. Top three pathways significantly altered between low vs. high CAA cerebrovessels included TCA Cycle II, Oxidative phosphorylation and mitochondrial dysfunction. Notably, high CAA cases included more advanced AD pathology thus cerebrovascular effects may be driven by the severity of amyloid and Tangle pathology. These descriptive proteomic changes provide novel insights to explain the age-related and AD-related cerebrovascular changes contributing to AD pathogenesis. Particularly, disturbances in energy bioenergetics and mitochondrial biology rank among the top AD pathways altered in cerebrovessels. Targeting these failed mechanisms in endothelia and mural cells may provide novel disease modifying targets for developing therapeutic strategies against cerebrovascular deterioration and promoting cerebral perfusion in AD. Our future work will focus on interrogating and validating these novel targets and pathways and their functional significance.
Keywords: Alzheimers disease; cerebral amyloid angiopathy; cerebrovasculature; endothelial cells; mass spectrometry; mural cells; perivascular cells; proteomics.
Copyright © 2021 Ojo, Reed, Crynen, Vallabhaneni, Evans, Shackleton, Eisenbaum, Ringland, Edsell, Mullan, Crawford and Bachmeier.
Conflict of interest statement
JR was employed by company Boehringer Ingelheim Pharmaceuticals, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
APOE genotype dependent molecular abnormalities in the cerebrovasculature of Alzheimer's disease and age-matched non-demented brains.Mol Brain. 2021 Jul 8;14(1):110. doi: 10.1186/s13041-021-00803-9. Mol Brain. 2021. PMID: 34238312 Free PMC article.
-
Proteomic Changes in the Human Cerebrovasculature in Alzheimer's Disease and Related Tauopathies Linked to Peripheral Biomarkers in Plasma and Cerebrospinal Fluid.medRxiv [Preprint]. 2024 Jan 11:2024.01.10.24301099. doi: 10.1101/2024.01.10.24301099. medRxiv. 2024. Update in: Alzheimers Dement. 2024 Jun;20(6):4043-4065. doi: 10.1002/alz.13821. PMID: 38260316 Free PMC article. Updated. Preprint.
-
Endothelial Mitochondrial Dysfunction in Cerebral Amyloid Angiopathy and Alzheimer's Disease.J Alzheimers Dis. 2019;72(4):1019-1039. doi: 10.3233/JAD-190357. J Alzheimers Dis. 2019. PMID: 31306129 Free PMC article. Review.
-
Reduced nitric oxide bioavailability mediates cerebroarterial dysfunction independent of cerebral amyloid angiopathy in a mouse model of Alzheimer's disease.Am J Physiol Heart Circ Physiol. 2017 Feb 1;312(2):H232-H238. doi: 10.1152/ajpheart.00607.2016. Epub 2016 Nov 11. Am J Physiol Heart Circ Physiol. 2017. PMID: 27836896
-
Potential Role of Venular Amyloid in Alzheimer's Disease Pathogenesis.Int J Mol Sci. 2020 Mar 14;21(6):1985. doi: 10.3390/ijms21061985. Int J Mol Sci. 2020. PMID: 32183293 Free PMC article. Review.
Cited by
-
Integrated Analysis of Cortex Single-Cell Transcriptome and Serum Proteome Reveals the Novel Biomarkers in Alzheimer's Disease.Brain Sci. 2022 Aug 1;12(8):1022. doi: 10.3390/brainsci12081022. Brain Sci. 2022. PMID: 36009085 Free PMC article.
-
Differences in the cerebral amyloid angiopathy proteome in Alzheimer's disease and mild cognitive impairment.Acta Neuropathol. 2024 Jul 22;148(1):9. doi: 10.1007/s00401-024-02767-1. Acta Neuropathol. 2024. PMID: 39039355 Free PMC article.
-
The elusive brain perivascular fibroblast: a potential role in vascular stability and homeostasis.Front Cardiovasc Med. 2023 Nov 24;10:1283434. doi: 10.3389/fcvm.2023.1283434. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 38075961 Free PMC article. Review.
-
Border-associated macrophages promote cerebral amyloid angiopathy and cognitive impairment through vascular oxidative stress.Res Sq [Preprint]. 2023 Apr 28:rs.3.rs-2719812. doi: 10.21203/rs.3.rs-2719812/v1. Res Sq. 2023. Update in: Mol Neurodegener. 2023 Oct 3;18(1):73. doi: 10.1186/s13024-023-00660-1. PMID: 37162996 Free PMC article. Updated. Preprint.
-
Capillary basal lamina in human brain and spinal cord has fibrillar collagen type I and type III: Ignorance may not be bliss.Free Neuropathol. 2025 Feb 18;6:6. doi: 10.17879/freeneuropathology-2025-6159. eCollection 2025 Jan. Free Neuropathol. 2025. PMID: 40012952 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

