Polypharmacy to Mitigate Acute and Delayed Radiation Syndromes
- PMID: 34079456
- PMCID: PMC8165380
- DOI: 10.3389/fphar.2021.634477
Polypharmacy to Mitigate Acute and Delayed Radiation Syndromes
Erratum in
-
Corrigendum: Polypharmacy to Mitigate Acute and Delayed Radiation Syndromes.Front Pharmacol. 2021 Aug 25;12:741485. doi: 10.3389/fphar.2021.741485. eCollection 2021. Front Pharmacol. 2021. PMID: 34512365 Free PMC article.
Abstract
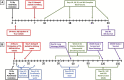
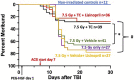
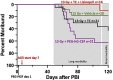
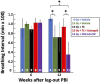
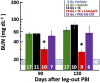
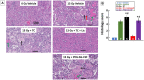
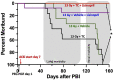
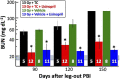
There is a need for countermeasures to mitigate lethal acute radiation syndrome (ARS) and delayed effects of acute radiation exposure (DEARE). In WAG/RijCmcr rats, ARS occurs by 30-days following total body irradiation (TBI), and manifests as potentially lethal gastrointestinal (GI) and hematopoietic (H-ARS) toxicities after >12.5 and >7 Gy, respectively. DEARE, which includes potentially lethal lung and kidney injuries, is observed after partial body irradiation >12.5 Gy, with one hind limb shielded (leg-out PBI). The goal of this study is to enhance survival from ARS and DEARE by polypharmacy, since no monotherapy has demonstrated efficacy to mitigate both sets of injuries. For mitigation of ARS following 7.5 Gy TBI, a combination of three hematopoietic growth factors (polyethylene glycol (PEG) human granulocyte colony-stimulating factor (hG-CSF), PEG murine granulocyte-macrophage-CSF (mGM-CSF), and PEG human Interleukin (hIL)-11), which have shown survival efficacy in murine models of H-ARS were tested. This triple combination (TC) enhanced survival by 30-days from ∼25% to >60%. The TC was then combined with proven medical countermeasures for GI-ARS and DEARE, namely enrofloxacin, saline and the angiotensin converting enzyme inhibitor, lisinopril. This combination of ARS and DEARE mitigators improved survival from GI-ARS, H-ARS, and DEARE after 7.5 Gy TBI or 13 Gy PBI. Circulating blood cell recovery as well as lung and kidney function were also improved by TC + lisinopril. Taken together these results demonstrate an efficacious polypharmacy to mitigate radiation-induced ARS and DEARE in rats.
Keywords: acute radiation syndrome; delayed effects of acute radiation exposure; hematopoietic growth factor; lisinopril; mitigation; polypharmacy; radiation pneumonitis; supportive care.
Copyright © 2021 Gasperetti, Miller, Gao, Narayanan, Jacobs, Szabo, Cox, Orschell, Fish and Medhora.
Conflict of interest statement
GC is an employee of Bolder BioTechnology, Inc. and has a financial interest in the company. GC and CO are inventors on patents related to use of PEG-HGFs to treat ARS. GC, CO, BF, and MM are inventors on a pending patent application related to the use of combinations of HGF and ACE inhibitors to treat ARS. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Amgen (2015b). Inc. “Neulasta Prescribing Information.”. Thousand Oaks, CA. Available at: http://pi.amgen.com/united_states/neulasta/neulasta_pi_hcp_english.pdf (Accessed March 28th, 2021).
-
- Amgen (2015a). Inc. “Neupogen Prescribing Information.”. Thousand Oaks, CA. Available at: http://pi.amgen.com/united_states/neupogen/neupogen_pi_hcp_english.pdf (Accessed March 28th, 2021).
-
- Amgen (2021). Inc. “Nplate Prescribing Information.”. Thousand Oaks, CA. Available at: https://www.pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/npla... (Accessed March 28th, 2021).
-
- Barshishat-Kupper M., Mungunsukh O., Tipton A. J., McCart E. A., Panganiban R. A. M., Davis T. A., et al. (2011). Captopril Modulates Hypoxia-Inducible Factors and Erythropoietin Responses in a Murine Model of Total Body Irradiation. Exp. Hematol. 39 (3), 293–304. 10.1016/j.exphem.2010.12.002 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

