Long-Acting Metformin Vs. Metformin Immediate Release in Patients With Type 2 Diabetes: A Systematic Review
- PMID: 34079464
- PMCID: PMC8165304
- DOI: 10.3389/fphar.2021.669814
Long-Acting Metformin Vs. Metformin Immediate Release in Patients With Type 2 Diabetes: A Systematic Review
Abstract
Background: Metformin, a commonly used antidiabetic medication, is available in both an immediate-release (IR) formulation and a long-acting formulation (metformin extended-release; XR). Objective: We performed a systematic review to compare the effectiveness, safety, and patient compliance and satisfaction between the metformin IR and XR formulations. Method: We searched for randomized control trials (RCTs) and observational studies comparing the effectiveness, safety, or patient compliance and satisfaction of metformin XR with metformin IR using the MEDLINE, Embase, and Cochrane Central Register of Controlled Trials databases. Following report screening, data collection, and risk of bias assessment, we separately pooled data from RCTs and observational studies using the Grading of Recommendation Assessment, Development, and Evaluation approach to rate the quality of evidence. Result: We included five RCTs, comprising a total of 1,662 patients, and one observational study, comprising 10,909 patients. In the meta-analyses, no differences were identified in outcomes of effectiveness and safety between the two forms of metformin (including change in HbA1c: mean difference (MD), 0.04%, 95% confidence interval [CI], -0.05-0.13%, fasting blood glucose: MD, -0.03 mmol/L, 95% CI, -0.22-0.15 mmol/L, postprandial blood glucose: MD, 0.50 mmol/L, 95% CI, -0.71-1.72 mmol/L, adverse events of abdominal pain: relative risk (RR), 1.15, 95% CI, 0.57-2.33, all-cause death (RR, 3.02, 95% CI 0.12-73.85), any adverse events (RR, 1.14, 95% CI 0.97-1.34), any adverse events leading to treatment discontinuation: RR, 1.51, 95% CI, 0.82-2.8, any gastrointestinal adverse events: RR, 1.09, 95% CI, 0.93-1.29, diarrhea: RR, 0.82, 95% CI, 0.53-1.27, flatulence: RR, 0.43, 95% CI, 0.15-1.23, nausea: RR, 0.97, 95% CI, 0.64-1.47, severe adverse events: RR, 0.64, 95% CI, 0.28-1.42, and vomiting: RR, 1.46, 95% CI, 0.6-3.56). Data from both the RCTs and the observational study indicate mildly superior patient compliance with metformin XR use compared with metformin IR use; this result was attributable to the preference for once-daily administration with metformin XR. Conclusion: Our systematic review indicates that metformin XR and IR formulations have similar effectiveness and safety, but that metformin XR is associated with improved compliance to treatment.
Keywords: meta-analysis; metformin extended-release; metformin immediate-release; once-daily consumption; patient value; systematic review; treatment compliance.
Copyright © 2021 Tan, Wang, Liu, Shi, Zhou, Zhou, Yang, Chen and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
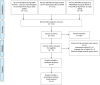

Similar articles
-
Efficacy and Side Effect Profile of Different Formulations of Metformin: A Systematic Review and Meta-Analysis.Diabetes Ther. 2021 Jul;12(7):1901-1914. doi: 10.1007/s13300-021-01058-2. Epub 2021 Jun 2. Diabetes Ther. 2021. PMID: 34075573 Free PMC article.
-
Comparative effectiveness of metformin monotherapy in extended release and immediate release formulations for the treatment of type 2 diabetes in treatment-naïve Chinese patients: Analysis of results from the CONSENT trial.Diabetes Obes Metab. 2018 Apr;20(4):1006-1013. doi: 10.1111/dom.13190. Epub 2018 Jan 22. Diabetes Obes Metab. 2018. PMID: 29227571 Clinical Trial.
-
Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: results of a retrospective cohort study.Curr Med Res Opin. 2004 Apr;20(4):565-72. doi: 10.1185/030079904125003278. Curr Med Res Opin. 2004. PMID: 15119994
-
Cochrane Review: Osmotic and stimulant laxatives for the management of childhood constipation (Review).Evid Based Child Health. 2013 Jan;8(1):57-109. doi: 10.1002/ebch.1893. Evid Based Child Health. 2013. PMID: 23878124 Review.
-
Gastrointestinal adverse events of metformin treatment in patients with type 2 diabetes mellitus: a systematic review and meta-analysis with meta-regression of observational studies.BMC Endocr Disord. 2024 Sep 30;24(1):206. doi: 10.1186/s12902-024-01727-w. BMC Endocr Disord. 2024. PMID: 39350158 Free PMC article.
Cited by
-
Improving Type 2 Diabetes Care with Extended-Release Metformin: Real-Life Insights from a Physician Educational Program.Endocr Metab Immune Disord Drug Targets. 2024;24(12):1422-1430. doi: 10.2174/0118715303294909240221102552. Endocr Metab Immune Disord Drug Targets. 2024. PMID: 38425116 Free PMC article.
-
Approved and Commercialized Antidiabetic Medicines (Excluding Insulin) in Seven European Countries-A Cross-Sectional Comparison.Pharmaceuticals (Basel). 2024 Jun 17;17(6):793. doi: 10.3390/ph17060793. Pharmaceuticals (Basel). 2024. PMID: 38931460 Free PMC article.
-
Metformin in Patients With COVID-19: A Systematic Review and Meta-Analysis.Front Med (Lausanne). 2021 Aug 19;8:704666. doi: 10.3389/fmed.2021.704666. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34490296 Free PMC article.
-
Pharmacological management of type 2 diabetes mellitus in children and adolescents: A systematic review and network meta-analysis.World J Diabetes. 2025 Jul 15;16(7):106890. doi: 10.4239/wjd.v16.i7.106890. World J Diabetes. 2025. PMID: 40697609 Free PMC article.
-
Comparison of the Efficacy and Safety of Metformin-Based Combination Therapy Versus Metformin Alone in Children and Adolescents With Type 2 Diabetes Mellitus: A Meta-Analysis.Cureus. 2023 Feb 15;15(2):e35014. doi: 10.7759/cureus.35014. eCollection 2023 Feb. Cureus. 2023. PMID: 36938239 Free PMC article. Review.
References
-
- Aggarwal N., Singla A., Mathieu C., Montanya E., Pfeiffer A. F. H., Johnsson E., et al. (2018). Metformin Extended‐release versus Immediate‐release: A N International, Randomized, Double‐blind, Head‐to‐head Trial in Pharmacotherapy‐naïve Patients with Type 2 Diabetes. Diabetes Obes. Metab. 20 (2), 463–467. 10.1111/dom.13104 - DOI - PMC - PubMed
-
- Amod A. (2012). The 2012 SEMDSA Guideline for the Management of Type 2 Diabetes. J. Endocrinol. Metab. Diabetes South Africa 17, 61–62. 10.1080/22201009.2012.10872276 - DOI
-
- Blonde L., Dailey G. E., Jabbour S. A., Reasner C. A., Mills D. J. (2004). Gastrointestinal Tolerability of Extended-Release Metformin Tablets Compared to Immediate-Release Metformin Tablets: Results of a Retrospective Cohort Study. Curr. Med. Res. Opin. 20 (4), 565–572. 10.1185/030079904125003278 - DOI - PubMed
LinkOut - more resources
Full Text Sources

