Effects of Treatment Setting on Outcomes of Flexibly-Dosed Intensive Cognitive Behavioral Therapy for Pediatric OCD: A Randomized Controlled Pilot Trial
- PMID: 34079488
- PMCID: PMC8165233
- DOI: 10.3389/fpsyt.2021.669494
Effects of Treatment Setting on Outcomes of Flexibly-Dosed Intensive Cognitive Behavioral Therapy for Pediatric OCD: A Randomized Controlled Pilot Trial
Abstract
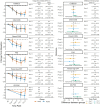
Introduction: Optimizing individual outcomes of cognitive-behavioral therapy (CBT) remains a priority. Methods: Youth were randomized to receive intensive CBT at a hospital clinic (n = 14) or within their home (n = 12). Youth completed 3 × 3 h sessions (Phase I) and up to four additional 3-h sessions as desired/needed (Phase II). An independent evaluator assessed youth after Phase I, Phase II (when applicable), and at 1- and 6-months post-treatment. A range of OCD-related (e.g., severity, impairment) and secondary (e.g., quality of life, comorbid symptoms) outcomes were assessed. Results: Families' satisfaction with the treatment program was high. Of study completers (n = 22), five youth (23%) utilized no Phase II sessions and 9 (41%) utilized all four (Median Phase II sessions: 2.5). Large improvements in OCD-related outcomes and small-to-moderate benefits across secondary domains were observed. Statistically-significant differences in primary outcomes were not observed between settings; however, minor benefits for home-based treatment were observed (e.g., maintenance of gains, youth comfort with treatment). Discussion: Intensive CBT is an efficacious treatment for pediatric OCD. Families opted for differing doses based on their needs. Home-based treatment, while not substantially superior to hospital care, may offer some value, particularly when desired/relevant. Clinical Trial Registration: www.ClinicalTrials.gov; https://clinicaltrials.gov/ct2/show/NCT03672565, identifier: NCT03672565.
Keywords: exposure and response prevention; family treatment; home-based treatment; stepped care; treatment trial.
Copyright © 2021 Selles, Naqqash, Best, Franco-Yamin, Qiu, Ferreira, Deng, Hannesdottir, Oberth, Belschner, Negreiros, Farrell and Stewart.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Freeman J, Benito K, Herren J, Kemp J, Sung J, Georgiadis C, et al. Evidence base update of psychosocial treatments for pediatric obsessive-compulsive disorder: evaluating, improving, and transporting what works. J Clin Child Adolesc Psychol. (2018) 47:669–98. 10.1080/15374416.2018.1496443 - DOI - PubMed
-
- McGuire JF, Piacentini J, Lewin AB, Brennan EA, Murphy TK, Storch EA. A meta-analysis of cognitive behavior therapy and medication for child obsessive-compulsive disorder: moderators of treatment efficacy, response, and remission. Depress Anxiety. (2015) 32:580–93. 10.1002/da.22389 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

