Pig Liver Esterases Hydrolyze Endocannabinoids and Promote Inflammatory Response
- PMID: 34079552
- PMCID: PMC8165269
- DOI: 10.3389/fimmu.2021.670427
Pig Liver Esterases Hydrolyze Endocannabinoids and Promote Inflammatory Response
Abstract
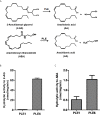
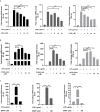
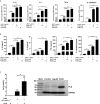
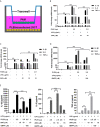
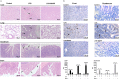
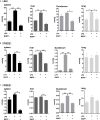
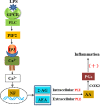
Endocannabinoids are endogenous ligands of cannabinoid receptors and activation of these receptors has strong physiological and pathological significance. Structurally, endocannabinoids are esters (e.g., 2-arachidonoylglycerol, 2-AG) or amides (e.g., N-arachidonoylethanolamine, AEA). Hydrolysis of these compounds yields arachidonic acid (AA), a major precursor of proinflammatory mediators such as prostaglandin E2. Carboxylesterases are known to hydrolyze esters and amides with high efficiency. CES1, a human carboxylesterase, has been shown to hydrolyze 2-AG, and shares a high sequence identity with pig carboxylesterases: PLE1 and PLE6 (pig liver esterase). The present study was designed to test the hypothesis that PLE1 and PLE6 hydrolyze endocannabinoids and promote inflammatory response. Consistent with the hypothesis, purified PLE1 and PLE6 efficaciously hydrolyzed 2-AG and AEA. PLE6 was 40-fold and 3-fold as active as PLE1 towards 2-AG and AEA, respectively. In addition, both PLE1 and PLE6 were highly sensitive to bis(4-nitrophenyl) phosphate (BNPP), an aryl phosphodiester known to predominately inhibit carboxylesterases. Based on the study with BNPP, PLEs contributed to the hydrolysis of 2-AG by 53.4 to 88.4% among various organs and cells. Critically, exogenous addition or transfection of PLE6 increased the expression and secretion of proinflammatory cytokines in response to the immunostimulant lipopolysaccharide (LPS). This increase was recapitulated in cocultured alveolar macrophages and PLE6 transfected cells in transwells. Finally, BNPP reduced inflammation trigged by LPS accompanied by reduced formation of AA and proinflammatory mediators. These findings define an innovative connection: PLE-endocannabinoid-inflammation. This mechanistic connection signifies critical roles of carboxylesterases in pathophysiological processes related to the metabolism of endocannabinoids.
Keywords: Carboxylesterases; arachidonic acid; endocannabinoid; inflammation; pig liver esterase; prostaglandins.
Copyright © 2021 Zhou, Yan, Sun, Chen, Xiao, Xiao, Wang and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Yan B. Hydrolytic Enzymes in Metabolism of Drugs and Other Xenobiotics. In: P Anzenbacher, UM Zanger, editors. Weinheim: WILEY-VCH Verlag GmbH & Co. KGaA; (2013). p. 165–98.
-
- Qiao Q, Bouwman FG, van Baak MA, Roumans NJT, Vink RG, Coort SLM, et al. Adipocyte Abundances of CES1, Cryab, ENO1 and GANAB are Modified in-Vitro by Glucose Restriction and are Associated With Cellular Remodelling During Weight Regain. Adipocyte (2019) 8:190–200. 10.1080/21623945.2019.1608757 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

