miRNA-Mediated Control of B Cell Responses in Immunity and SLE
- PMID: 34079558
- PMCID: PMC8165268
- DOI: 10.3389/fimmu.2021.683710
miRNA-Mediated Control of B Cell Responses in Immunity and SLE
Abstract
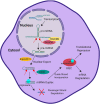
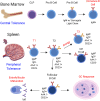
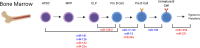
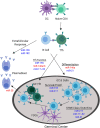
Loss of B cell tolerance is central to autoimmune diseases such as systemic lupus erythematosus (SLE). As such, the mechanisms involved in B cell development, maturation, activation, and function that are aberrantly regulated in SLE are of interest in the design of targeted therapeutics. While many factors are involved in the generation and regulation of B cell responses, miRNAs have emerged as critical regulators of these responses within the last decade. To date, miRNA involvement in B cell responses has largely been studied in non-autoimmune, immunization-based systems. However, miRNA profiles have also been strongly associated with SLE in human patients and these molecules have proven critical in both the promotion and regulation of disease in mouse models and in the formation of autoreactive B cell responses. Functionally, miRNAs are small non-coding RNAs that bind to complementary sequences located in target mRNA transcripts to mediate transcript degradation or translational repression, invoking a post-transcriptional level of genetic regulation. Due to their capacity to target a diverse range of transcripts and pathways in different immune cell types and throughout the various stages of development and response, targeting miRNAs is an interesting potential therapeutic avenue. Herein, we focus on what is currently known about miRNA function in both normal and SLE B cell responses, primarily highlighting miRNAs with confirmed functions in mouse models. We also discuss areas that should be addressed in future studies and whether the development of miRNA-centric therapeutics may be a viable alternative for the treatment of SLE.
Keywords: B cells; autoimmunity; germinal center; miRNA; systemic lupus erythematosus.
Copyright © 2021 Schell and Rahman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Role of miRNAs in Apoptosis Pathways of Immune Cells in Systemic Lupus Erythematosus.Immun Inflamm Dis. 2025 Feb;13(2):e70124. doi: 10.1002/iid3.70124. Immun Inflamm Dis. 2025. PMID: 39912562 Free PMC article. Review.
-
Regulation of B-cell function by miRNAs impacting Systemic lupus erythematosus progression.Gene. 2025 Jan 15;933:149011. doi: 10.1016/j.gene.2024.149011. Epub 2024 Oct 18. Gene. 2025. PMID: 39427831 Review.
-
Selective Histone Deacetylase 6 Inhibition Normalizes B Cell Activation and Germinal Center Formation in a Model of Systemic Lupus Erythematosus.Front Immunol. 2019 Oct 25;10:2512. doi: 10.3389/fimmu.2019.02512. eCollection 2019. Front Immunol. 2019. PMID: 31708928 Free PMC article.
-
Decreased microRNA(miR)-145 and increased miR-224 expression in T cells from patients with systemic lupus erythematosus involved in lupus immunopathogenesis.Clin Exp Immunol. 2013 Jan;171(1):91-9. doi: 10.1111/j.1365-2249.2012.04676.x. Clin Exp Immunol. 2013. PMID: 23199328 Free PMC article.
-
Recent Advances in Lupus B Cell Biology: PI3K, IFNγ, and Chromatin.Front Immunol. 2021 Jan 14;11:615673. doi: 10.3389/fimmu.2020.615673. eCollection 2020. Front Immunol. 2021. PMID: 33519824 Free PMC article. Review.
Cited by
-
Human immune system: Exploring diversity across individuals and populations.Heliyon. 2025 Jan 13;11(2):e41836. doi: 10.1016/j.heliyon.2025.e41836. eCollection 2025 Jan 30. Heliyon. 2025. PMID: 39911431 Free PMC article. Review.
-
MicroRNA as a potential biomarker for systemic lupus erythematosus: pathogenesis and targeted therapy.Clin Exp Med. 2023 Dec;23(8):4065-4077. doi: 10.1007/s10238-023-01234-7. Epub 2023 Nov 3. Clin Exp Med. 2023. PMID: 37921874 Review.
-
UVB irradiation differential regulate miRNAs expression in skin photoaging.An Bras Dermatol. 2022 Jul-Aug;97(4):458-466. doi: 10.1016/j.abd.2022.01.003. Epub 2022 May 31. An Bras Dermatol. 2022. PMID: 35660030 Free PMC article.
-
The new era of immune skin diseases: Exploring advances in basic research and clinical translations.J Transl Autoimmun. 2024 Jan 12;8:100232. doi: 10.1016/j.jtauto.2024.100232. eCollection 2024 Jun. J Transl Autoimmun. 2024. PMID: 39022635 Free PMC article. No abstract available.
-
Deletion of microRNA-183-96-182 Cluster in Lymphocytes Suppresses Anti-DsDNA Autoantibody Production and IgG Deposition in the Kidneys in C57BL/6-Faslpr/lpr Mice.Front Genet. 2022 Jul 7;13:840060. doi: 10.3389/fgene.2022.840060. eCollection 2022. Front Genet. 2022. PMID: 35873462 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

