PD-1-Positive Tumor-Associated Macrophages Define Poor Clinical Outcomes in Patients With Muscle Invasive Bladder Cancer Through Potential CD68/PD-1 Complex Interactions
- PMID: 34079767
- PMCID: PMC8165482
- DOI: 10.3389/fonc.2021.679928
PD-1-Positive Tumor-Associated Macrophages Define Poor Clinical Outcomes in Patients With Muscle Invasive Bladder Cancer Through Potential CD68/PD-1 Complex Interactions
Abstract
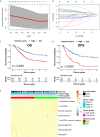
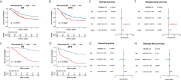
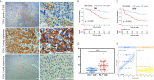
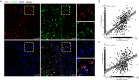
Tumor-associated macrophages (TAMs) regulate tumor immunity. Previous studies have shown that the programmed cell death protein 1 (PD-1)-positive TAMs have an M2 macrophage phenotype. CD68 is a biomarker of TAMs and is considered to be a poor prognostic marker of several malignancies. Our results show that PD-1-positive TAMs can be a negative survival indicator in patients with muscle-invasive bladder cancer (MIBC), and that the mechanistic effects could result due to a combination of PD-1 and CD68 activity. We analyzed 22 immune cell types using data from 402 patients with MIBC from the TCGA database, and found that a high immune score and M2 TAMs were strongly associated with poor clinical outcomes in patients with MIBC. Further, we analyzed resected samples from 120 patients with MIBC and found that individuals with PD-1-positive TAMs showed a reduction in 5-year overall survival and disease-free survival. Additionally, PD-1-positive TAMs showed a significant association with higher programmed death-ligand 1 (PD-L1) expression, the Ki67 index, the pT stage and fewer CD8-positive T cells. Through the co-immunoprecipitation (co-IP) assay of THP-1 derived macrophages, we found that CD68 can bind to PD-1. The binding of CD68 and PD-1 can induce M2 polarization of THP-1 derived macrophages and promote cancer growth. The anti-CD68 treatment combined with peripheral blood mononuclear cells (PBMC) showed obvious synergy effects on inhibiting the proliferation of T24 cells. Together, these results indicate for the first time that CD68/PD-1 may be a novel target for the prognosis of patients with MIBC.
Keywords: CD68; PD-1; muscle-invasive bladder cancer; prognosis; tumor-associated macrophages.
Copyright © 2021 Jiang, Zhang, Chen, He, Liu, Han, Shi, Yang, Mu, Fu and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Dynamic alteration and prognostic significance of tumor-associated CD68+ and CD68+ PD-L1- macrophages in muscle-invasive bladder cancer treated with neoadjuvant chemotherapy.Cancer Med. 2023 Feb;12(4):4981-4992. doi: 10.1002/cam4.5191. Epub 2022 Aug 31. Cancer Med. 2023. PMID: 36043478 Free PMC article.
-
Poor clinical outcomes and immunoevasive contexture in SIRPα+ tumor-associated macrophages enriched muscle-invasive bladder cancer patients.Urol Oncol. 2022 Mar;40(3):109.e11-109.e20. doi: 10.1016/j.urolonc.2021.08.024. Epub 2021 Sep 30. Urol Oncol. 2022. PMID: 34600802
-
Blockade of DC-SIGN+ Tumor-Associated Macrophages Reactivates Antitumor Immunity and Improves Immunotherapy in Muscle-Invasive Bladder Cancer.Cancer Res. 2020 Apr 15;80(8):1707-1719. doi: 10.1158/0008-5472.CAN-19-2254. Epub 2020 Feb 14. Cancer Res. 2020. PMID: 32060149
-
Prognostic Impact of Tumor-Associated Macrophages on Survival Is Checkpoint Dependent in Classical Hodgkin Lymphoma.Cancers (Basel). 2020 Apr 4;12(4):877. doi: 10.3390/cancers12040877. Cancers (Basel). 2020. PMID: 32260340 Free PMC article.
-
Triple negative breast cancer: Key role of Tumor-Associated Macrophages in regulating the activity of anti-PD-1/PD-L1 agents.Biochim Biophys Acta Rev Cancer. 2018 Jan;1869(1):78-84. doi: 10.1016/j.bbcan.2017.10.007. Epub 2017 Nov 7. Biochim Biophys Acta Rev Cancer. 2018. PMID: 29126881 Review.
Cited by
-
SYNPO2 promotes the development of BLCA by upregulating the infiltration of resting mast cells and increasing the resistance to immunotherapy.Oncol Rep. 2024 Jan;51(1):14. doi: 10.3892/or.2023.8673. Epub 2023 Dec 1. Oncol Rep. 2024. PMID: 38038167 Free PMC article.
-
The Prognostic Significance of CD47, CD68, and CD163 Expression Levels and Their Relationship with MLR and MAR in Locally Advanced and Oligometastatic Nasopharyngeal Carcinoma.Diagnostics (Basel). 2024 Nov 24;14(23):2648. doi: 10.3390/diagnostics14232648. Diagnostics (Basel). 2024. PMID: 39682556 Free PMC article.
-
Tumor-Associated Macrophages: Key Players in Triple-Negative Breast Cancer.Front Oncol. 2022 Feb 14;12:772615. doi: 10.3389/fonc.2022.772615. eCollection 2022. Front Oncol. 2022. PMID: 35237507 Free PMC article. Review.
-
The feasibility of proteomics sequencing based immune-related prognostic signature for predicting clinical outcomes of bladder cancer patients.BMC Cancer. 2022 Jun 20;22(1):676. doi: 10.1186/s12885-022-09783-y. BMC Cancer. 2022. PMID: 35725413 Free PMC article.
-
A novel signature constructed by ferroptosis-associated genes (FAGs) for the prediction of prognosis in bladder urothelial carcinoma (BLCA) and associated with immune infiltration.Cancer Cell Int. 2021 Aug 6;21(1):414. doi: 10.1186/s12935-021-02096-3. Cancer Cell Int. 2021. PMID: 34362387 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

