The Masking Game: Design of Activatable Antibodies and Mimetics for Selective Therapeutics and Cell Control
- PMID: 34079893
- PMCID: PMC8161478
- DOI: 10.1021/acscentsci.0c01448
The Masking Game: Design of Activatable Antibodies and Mimetics for Selective Therapeutics and Cell Control
Abstract
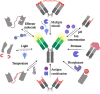
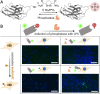
The high selectivity and affinity of antibody binding have made antibodies all-pervasive tools in therapy, diagnosis, and basic science. A plethora of chemogenetic approaches has been devised to make antibodies responsive to stimuli ranging from light to enzymatic activity, temperature, pH, ions, and effector molecules. Within a single decade, the field of activatable antibodies has yielded marketed therapeutics capable of engaging antigens that could not be targeted with traditional antibodies, as well as new tools to control intracellular protein location and investigate biological processes. Many opportunities remain untapped, waiting for more efficient and generally applicable masking strategies to be developed at the interface between chemistry and biotechnology.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Meulenberg E. P.Antibodies Applications and New Development; Meulenberg E. P., Ed.; Bentham Science Publishers Ltd., 2012.
-
- Wen J.; Wu D.; Qin M.; Liu C.; Wang L.; Xu D.; Vinters H. V.; Liu Y.; Kranz E.; Guan X.; Sun G.; Sun X.; Lee Y.; Martinez-Maza O.; Widney D.; Lu Y.; Chen I. S.; Kamata M. Sustained Delivery and Molecular Targeting of a Therapeutic Monoclonal Antibody to Metastases in the Central Nervous System of Mice. Nat. Biomed Eng. 2019, 3 (9), 706–716. 10.1038/s41551-019-0434-z. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

