Synthetic Organic "Aquachemistry" that Relies on Neither Cosolvents nor Surfactants
- PMID: 34079894
- PMCID: PMC8161484
- DOI: 10.1021/acscentsci.1c00045
Synthetic Organic "Aquachemistry" that Relies on Neither Cosolvents nor Surfactants
Abstract
There is a growing awareness of the underlying power of catalytic reactions in water that is not limited to innate sustainability alone. Some Type III reactions are catalytically accelerated without dissolution of reactants and are occasionally highly selective, as shown by comparison with the corresponding reactions run in organic solvents or under solvent-free conditions. Such catalysts are highly diversified, including hydrophilic, lipophilic, and even solid catalysts. In this Outlook, we highlight the impressive characteristics of illustrative catalysis that is exerted despite the immiscibility of the substrates and reveal the intrinsic benefits of these enigmatic reactions for synthetic organic chemistry, albeit with many details remaining unclear. We hope that this brief introduction to the expanding field of synthetic organic "aquachemistry" will inspire organic chemists to use the platform to invent new transformations.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
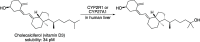
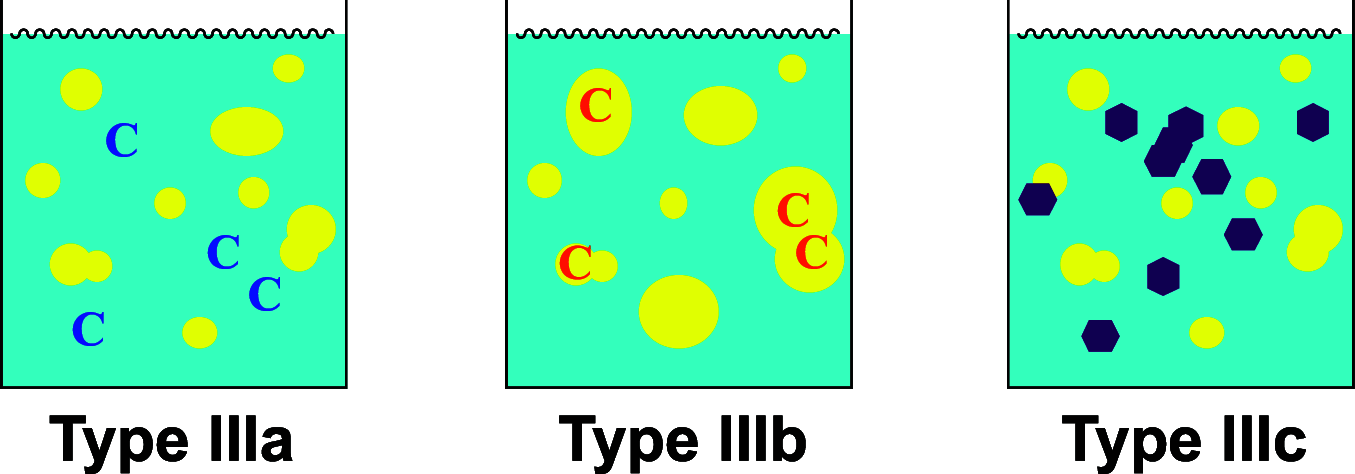
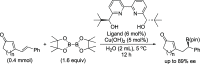
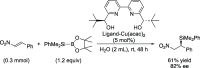
References
-
-
“The substances do not react unless fluid or if dissolved”, quoted by Aristotle (384–322 BCE).
-
-
- Cortes-Clerget M.; Yu J.; Kincaid J. R. A.; Walde P.; Gallou F.; Lipshutz B. H. Water as the reaction medium in organic chemistry: from our worst enemy to our best friend. Chem. Sci. 2021, 12, 4237. 10.1039/D0SC06000C. - DOI - PMC - PubMed
- Zhou F.; Hearne Z.; Li C.-J. Water–the greenest solvent overall. Curr. Opin. Green Sustain. Chem. 2019, 18, 118–123. 10.1016/j.cogsc.2019.05.004. - DOI
- Kitanosono T.; Masuda K.; Xu P.; Kobayashi S. Catalytic organic reactions in water toward sustainable society. Chem. Rev. 2018, 118, 679–746. 10.1021/acs.chemrev.7b00417. - DOI - PubMed
-
and references cited therein.
-
-
Recent authoritative reviews, see:
- Andersen J.; Mack J. Mechanochemistry and organic synthesis: from mystical to practical. Green Chem. 2018, 20, 1435–1443. 10.1039/C7GC03797J. - DOI
- Tan D.; Friščić T. Mechanochemistry for organic chemists: an update. Eur. J. Org. Chem. 2018, 2018, 18–33. 10.1002/ejoc.201700961. - DOI
- Do J.-L.; Friščić T. Mechanochemistry: a force of synthesis. ACS Cent. Sci. 2017, 3, 13–19. 10.1021/acscentsci.6b00277. - DOI - PMC - PubMed
-
and references cited therein.
-
-
- Wöhler F. Ueber künstliche Bildung des Harnstoffs. Ann. Phys. 1828, 87, 253–256. 10.1002/andp.18280870206. - DOI
Publication types
LinkOut - more resources
Full Text Sources

