Continuum Crystallization Model Derived from Pharmaceutical Crystallization Mechanisms
- PMID: 34079905
- PMCID: PMC8161475
- DOI: 10.1021/acscentsci.1c00254
Continuum Crystallization Model Derived from Pharmaceutical Crystallization Mechanisms
Abstract
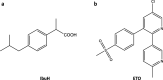
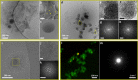
The crystallization mechanisms of organic molecules in solution are not well-understood. The mechanistic scenarios where crystalline order evolves directly from the molecularly dissolved state ("classical") and from initially formed amorphous intermediates ("nonclassical") are suggested and debated. Here, we studied crystallization mechanisms of two widely used analgesics, ibuprofen (IbuH) and etoricoxib (ETO), using direct cryogenic transmission electron microscopy (cryo-TEM) imaging. In the IbuH case, parallel crystallization pathways involved diverse phases of high and low density, in which the instantaneous formation of final crystalline order was observed. ETO crystallization started from well-defined round-shaped amorphous intermediates that gradually evolved into crystals. This mechanistic diversity is rationalized by introducing a continuum crystallization paradigm: order evolution depends on ordering in the initially formed intermediates and efficiency of molecular rearrangements within them, and there is a continuum of states related to the initial order and rearrangement rates. This model provides a unified view of crystallization mechanisms, encompassing classical and nonclassical pictures.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Multistep Crystallization of Pharmaceutical Amorphous Nanoparticles via a Cognate Pathway of Oriented Attachment: Direct Evidence of Nonclassical Crystallization for Organic Molecules.Nano Lett. 2022 Aug 24;22(16):6841-6846. doi: 10.1021/acs.nanolett.2c01608. Epub 2022 Jul 13. Nano Lett. 2022. PMID: 35830610
-
Crystallization of Organic Molecules: Nonclassical Mechanism Revealed by Direct Imaging.ACS Cent Sci. 2018 Aug 22;4(8):1031-1036. doi: 10.1021/acscentsci.8b00289. Epub 2018 Jul 27. ACS Cent Sci. 2018. PMID: 30159400 Free PMC article.
-
A mechanism of ferritin crystallization revealed by cryo-STEM tomography.Nature. 2020 Mar;579(7800):540-543. doi: 10.1038/s41586-020-2104-4. Epub 2020 Mar 25. Nature. 2020. PMID: 32214264
-
Direct Imaging of the Kinetic Crystallization Pathway: Simulation and Liquid-Phase Transmission Electron Microscopy Observations.Materials (Basel). 2023 Mar 1;16(5):2026. doi: 10.3390/ma16052026. Materials (Basel). 2023. PMID: 36903141 Free PMC article. Review.
-
Nonclassical crystallization in vivo et in vitro (II): Nanogranular features in biomimetic minerals disclose a general colloid-mediated crystal growth mechanism.J Struct Biol. 2016 Nov;196(2):260-287. doi: 10.1016/j.jsb.2016.09.005. Epub 2016 Sep 9. J Struct Biol. 2016. PMID: 27620641 Review.
Cited by
-
Amorphous aggregates with a very wide size distribution play a central role in crystal nucleation.Chem Sci. 2024 Jul 4;15(31):12420-12430. doi: 10.1039/d4sc00452c. eCollection 2024 Aug 7. Chem Sci. 2024. PMID: 39118639 Free PMC article.
-
The rise and fall of adenine clusters in the gas phase: a glimpse into crystal growth and nucleation.Anal Bioanal Chem. 2024 Sep;416(23):5037-5048. doi: 10.1007/s00216-024-05442-2. Epub 2024 Jul 20. Anal Bioanal Chem. 2024. PMID: 39031229
-
Nonclassical Crystallization and Core-Shell Structure Formation of Ibuprofen from Binary Solvent Solutions.Cryst Growth Des. 2023 Jan 4;23(1):236-245. doi: 10.1021/acs.cgd.2c00971. Epub 2022 Dec 21. Cryst Growth Des. 2023. PMID: 36624777 Free PMC article.
-
Rationalizing the Influence of Solvent on the Nucleation of Griseofulvin through Classical and Nonclassical Pathways.Cryst Growth Des. 2025 Jun 3;25(13):4713-4724. doi: 10.1021/acs.cgd.5c00206. eCollection 2025 Jul 2. Cryst Growth Des. 2025. PMID: 40625785 Free PMC article.
-
Nucleation of glucose isomerase protein crystals in a nonclassical disguise: The role of crystalline precursors.Proc Natl Acad Sci U S A. 2022 Feb 15;119(7):e2108674119. doi: 10.1073/pnas.2108674119. Proc Natl Acad Sci U S A. 2022. PMID: 35101915 Free PMC article.
References
-
- Grotzinger J. P.; James N. P.. Carbonate Sedimentation and Diagenesis in the Evolving Precambrian World; Grotzinger J. P., James N. P., Eds.; Society for Sedimentary Geology, 2000.
LinkOut - more resources
Full Text Sources

