The hen's egg test for micronucleus induction (HET-MN): validation data set
- PMID: 34080017
- PMCID: PMC9071061
- DOI: 10.1093/mutage/geab016
The hen's egg test for micronucleus induction (HET-MN): validation data set
Abstract
The classical in vitro genotoxicity test battery is known to be sensitive for indicating genotoxicity. However, a high rate of 'misleading positives' was reported when three assays were combined as required by several legislations. Despite the recent optimisations of the standard in vitro tests, two gaps could hardly be addressed with assays based on 2D monolayer cell cultures: the route of exposure and a relevant intrinsic metabolic capacity to transform pro-mutagens into reactive metabolites. Following these considerations, fertilised chicken eggs have been introduced into genotoxicity testing and were combined with a classical read-out parameter, the micronucleus frequency in circulating erythrocytes, to develop the hen's egg test for micronucleus induction (HET-MN). As a major advantage, the test mirrors the systemic availability of compounds after oral exposure by reflecting certain steps of Absorption, Distribution, Metabolism, Excretion (ADME) without being considered as an animal experiment. The assay is supposed to add to a toolbox of assays to follow up on positive findings from initial testing with classical in vitro assays. We here report on a validation exercise, in which >30 chemicals were tested double-blinded in three laboratories. The specificity and sensitivity of the HET-MN were calculated to be 98 and 84%, respectively, corresponding to an overall accuracy of 91%. A detailed protocol, which includes a picture atlas detailing the cell and micronuclei analysis, is published in parallel (Maul et al. Validation of the hen's egg test for micronucleus induction (HET-MN): detailed protocol including scoring atlas, historical control data and statistical analysis).
© The Author(s) 2021. Published by Oxford University Press on behalf of the UK Environmental Mutagen Society.
Figures
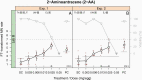
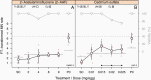

Similar articles
-
Validation of the hen's egg test for micronucleus induction (HET-MN): detailed protocol including scoring atlas, historical control data and statistical analysis.Mutagenesis. 2022 May 4;37(2):76-88. doi: 10.1093/mutage/geab026. Mutagenesis. 2022. PMID: 34313790 Free PMC article.
-
Hen's Egg Test for Micronucleus Induction (HET-MN).Methods Mol Biol. 2019;2031:195-208. doi: 10.1007/978-1-4939-9646-9_10. Methods Mol Biol. 2019. PMID: 31473961
-
The hen's egg test for micronucleus induction (HET-MN): novel analyses with a series of well-characterized substances support the further evaluation of the test system.Mutat Res. 2008 Feb 29;650(2):150-64. doi: 10.1016/j.mrgentox.2007.11.009. Epub 2007 Dec 15. Mutat Res. 2008. PMID: 18201925
-
Application of the in vitro rat hepatocyte micronucleus assay in genetic toxicology testing.Mutat Res. 1997 Aug 1;392(1-2):125-38. doi: 10.1016/s0165-1218(97)00051-7. Mutat Res. 1997. PMID: 9269337 Review.
-
Does the recommended lymphocyte cytokinesis-block micronucleus assay for human biomonitoring actually detect DNA damage induced by occupational and environmental exposure to genotoxic chemicals?Mutagenesis. 2013 Jul;28(4):375-80. doi: 10.1093/mutage/get026. Epub 2013 May 3. Mutagenesis. 2013. PMID: 23644166 Review.
Cited by
-
Validation of the hen's egg test for micronucleus induction (HET-MN): detailed protocol including scoring atlas, historical control data and statistical analysis.Mutagenesis. 2022 May 4;37(2):76-88. doi: 10.1093/mutage/geab026. Mutagenesis. 2022. PMID: 34313790 Free PMC article.
References
-
- OECD (2016) Test No. 487: In Vitro Mammalian Cell Micronucleus Test. OECD Publishing, Paris, France.
-
- SCCS (2018) The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation 10th Revision. SCCS/1602/18. https://ec.europa.eu/health/sites/health/files/scientific_committees/con....
-
- EU (2006) Regulation EC No. 1907/2006 of the European Parliament and of the Council of Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. https://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:136:0003....
-
- EU (2009) Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R1107....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

