Magnetic resonance imaging in neuromyelitis optica spectrum disorder
- PMID: 34080180
- PMCID: PMC8561702
- DOI: 10.1111/cei.13630
Magnetic resonance imaging in neuromyelitis optica spectrum disorder
Abstract
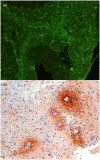
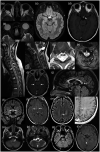
Neuromyelitis optica spectrum disorder (NMOSD) is an inflammatory disease of the central nervous system (CNS) associated with antibodies to aquaporin-4 (AQP4), which has distinct clinical, radiological and pathological features, but also has some overlap with multiple sclerosis and myelin oligodendrocyte glycoprotein (MOG) antibody associated disease. Early recognition of NMOSD is important because of differing responses to both acute and preventive therapy. Magnetic resonance (MR) imaging has proved essential in this process. Key MR imaging clues to the diagnosis of NMOSD are longitudinally extensive lesions of the optic nerve (more than half the length) and spinal cord (three or more vertebral segments), bilateral optic nerve lesions and lesions of the optic chiasm, area postrema, floor of the IV ventricle, periaqueductal grey matter, hypothalamus and walls of the III ventricle. Other NMOSD-specific lesions are denoted by their unique morphology: heterogeneous lesions of the corpus callosum, 'cloud-like' gadolinium (Gd)-enhancing white matter lesions and 'bright spotty' lesions of the spinal cord. Other lesions described in NMOSD, including linear periventricular peri-ependymal lesions and patch subcortical white matter lesions, may be less specific. The use of advanced MR imaging techniques is yielding further useful information regarding focal degeneration of the thalamus and optic radiation in NMOSD and suggests that paramagnetic rim patterns and changes in normal appearing white matter are specific to MS. MR imaging is crucial in the early recognition of NMOSD and in directing testing for AQP4 antibodies and guiding immediate acute treatment decisions. Increasingly, MR imaging is playing a role in diagnosing seronegative cases of NMOSD.
Keywords: diagnosis; magnetic resonance imaging; multiple sclerosis; neuromyelitis optica.
© 2021 British Society for Immunology.
Conflict of interest statement
Simon A. Broadley has received honoraria for attendance at advisory boards and travel sponsorship from Bayer‐Schering, Biogen‐Idec, Merck‐Serono, Novartis and Sanofi‐Genzyme, has received speaker’s honoraria from Biogen‐Idec and Genzyme, is an investigator in clinical trials sponsored by Biogen Idec, Novartis and Genzyme and was the recipient of an unencumbered research grant from Biogen‐Idec. Laura Clarke, Simon Arnett, Kate Lilley, Jacky Liao and Sandeep Bhuta report no disclosures.
Figures

References
-
- Devic E. Myélite subaiguë compliquée de névrite optique. Bull Med. 1894;8:1033–4.
-
- Devic E. Acute lumbar spinal myelitis with optic neuritis, autopsy. French Cong Med. 1895;1:434–9.
-
- Albutt FC. On the ophthalmoscopic signs of spinal cord disease. Lancet 1870;i:76–8.
-
- Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

