The diagnostic accuracy of saliva testing for SARS-CoV-2: A systematic review and meta-analysis
- PMID: 34080272
- PMCID: PMC8242702
- DOI: 10.1111/odi.13934
The diagnostic accuracy of saliva testing for SARS-CoV-2: A systematic review and meta-analysis
Abstract
Introduction: Early detection of coronavirus disease 2019 (COVID-19) is paramount for controlling the progression and spread of the disease. Currently, nasopharyngeal swabbing (NPS) is the standard method for collecting specimens. Saliva was recently proposed as an easy and safe option with many authorities adopting the methodology despite the limited evidence of efficacy.
Objectives: The aim of this review was to systematically evaluate the current literature on the use of saliva test for detecting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and carry out a meta-analysis to determine its diagnostic accuracy.
Materials and methods: Prospective studies were searched for in electronic databases, complemented by hand-searching relevant journals. The risk of bias and applicability were assessed using the revised Quality Assessment of Studies of Diagnostic Accuracy Studies (QUADAS-2) tool. Meta-analyses and meta-regression modeling were performed to calculate the diagnostic accuracy and examine sources of heterogeneity.
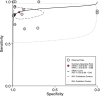
Results: A total of 16 studies were included with 2928 paired samples. The overall meta-analysis showed a high sensitivity and specificity for saliva test at 0.88 (95% CI 0.82-0.92) and 0.92 (95% CI 0.75-0.98), respectively. The diagnostic odds ratio was calculated at 87 (95% CI 19-395) and area under the curve was calculated as 0.92 (95% CI 0.90-0.94) suggesting very good performance of the saliva tests in detecting SARS-CoV-2.
Conclusion: Saliva testing has a very good discriminative and diagnostic ability to detect of SARS-CoV-2. Additional large and well-designed prospective studies are needed to further validate the diagnostic accuracy and determine a safe sample collection method prior to its recommendation for mass application.
Clinical relevance: Saliva demonstrated high sensitivity and specificity. The use of saliva will allow for self-collection of specimens and specimen collection in outpatient and community clinics.
Keywords: coronavirus disease; detection; review; saliva; severe acute respiratory syndrome coronavirus 2.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
Momen Atieh declares that he has no conflict of interest. Marina Guirguis declares that she has no conflict of interest. Nabeel Alsabeeha declares that he has no conflict of interest. Richard Cannon declares that he has no conflict of interest.
Figures






References
-
- Aita, A. , Basso, D. , Cattelan, A. M. , Fioretto, P. , Navaglia, F. , Barbaro, F. , Stoppa, A. , Coccorullo, E. , Farella, A. , Socal, A. , Vettor, R. , & Plebani, M. (2020). SARS‐CoV‐2 identification and IgA antibodies in saliva: One sample two tests approach for diagnosis. Clinica Chimica Acta, 510, 717–722. 10.1016/j.cca.2020.09.018 - DOI - PMC - PubMed
-
- Berenger, B. M. , Conly, J. M. , Fonseca, K. , Hu, J. , Louie, T. , Schneider, A. R. , Singh, T. , Stokes, W. , Ward, L. , & Zelyas, N. (2021). Saliva collected in universal transport media is an effective, simple and high‐volume amenable method to detect SARS‐CoV‐2. Clinical Microbiology and Infection, 27(4), 656–657. 10.1016/j.cmi.2020.10.035 - DOI - PMC - PubMed
-
- Binder, R. A. , Alarja, N. A. , Robie, E. R. , Kochek, K. E. , Xiu, L. , Rocha‐Melogno, L. , Abdelgadir, A. , Goli, S. V. , Farrell, A. S. , Coleman, K. K. , Turner, A. L. , Lautredou, C. C. , Lednicky, J. A. , Lee, M. J. , Polage, C. R. , Simmons, R. A. , Deshusses, M. A. , Anderson, B. D. , & Gray, G. C. (2020). Environmental and aerosolized severe acute respiratory syndrome coronavirus 2 among hospitalized coronavirus disease 2019 patients. Journal of Infectious Diseases, 222(11), 1798–1806. 10.1093/infdis/jiaa575 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

