Piperazine skeleton in the structural modification of natural products: a review
- PMID: 34080510
- PMCID: PMC8183565
- DOI: 10.1080/14756366.2021.1931861
Piperazine skeleton in the structural modification of natural products: a review
Abstract
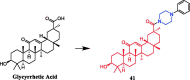
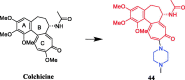
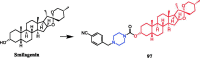
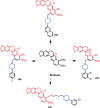
Piperazine moiety is a cyclic molecule containing two nitrogen atoms in positions 1 and 4, as well as four carbon atoms. Piperazine is one of the most sought heterocyclics for the development of new drug candidates with a wide range of applications. Over 100 molecules with a broad range of bioactivities, including antitumor, antibacterial, anti-inflammatory, antioxidant, and other activities, were reviewed. This article reviewed investigations regarding piperazine groups for the modification of natural product derivatives in the last decade, highlighting parameters that affect their biological activity.
Keywords: Piperazine; natural product; pharmacological activity; structure–activity relationship.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
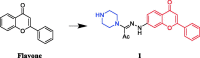
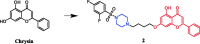
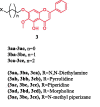
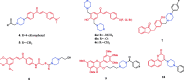



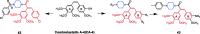
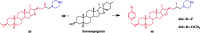
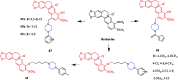

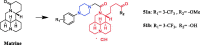
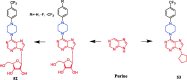

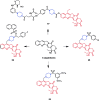

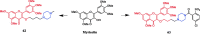
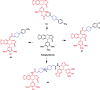
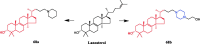
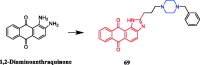
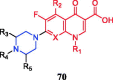
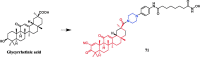
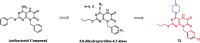
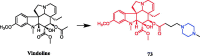
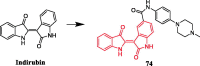
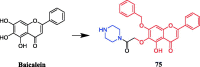

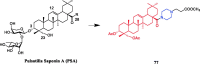
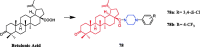
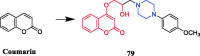
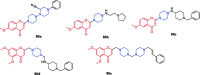
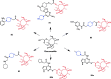
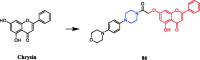
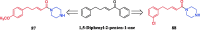
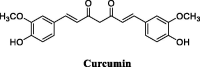
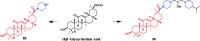
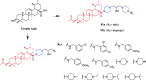
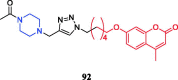
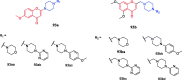
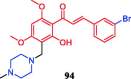
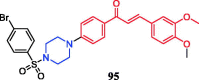

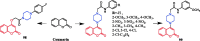
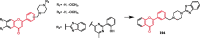
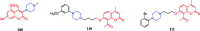
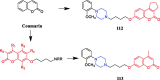
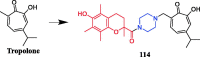
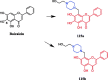



References
-
- Shaquiquzzaman M, Verma G, Marella A, et al. Piperazine scaffold: a remarkable tool in generation of diverse pharmacological agents. Eur J Med Chem 2015;102:487–529. - PubMed
-
- Chen F-H, Zhang L-B, Qiang L, et al. Reactive oxygen species-mitochondria pathway involved in LYG-202-induced apoptosis in human hepatocellular carcinoma HepG(2) cells. Cancer Lett 2010;296:96–105. - PubMed
-
- Foley TL, Rai G, Yasgar A, et al. 4-(3-Chloro-5-(trifluoromethyl)pyridin-2-yl)-N-(4- methoxypyridin-2-yl)piperazine-1-carbothioamide (ML267), a potent inhibitor of bacterial phosphopantetheinyl transferase that attenuates secondary metabolism and thwarts bacterial growth. J Med Chem 2014;57:1063–78. - PMC - PubMed
-
- Cao X, Zhang Y, Chen Y, et al. Synthesis and biological evaluation of fused tricyclic heterocycle piperazine (piperidine) derivatives as potential multireceptor atypical antipsychotics. J Med Chem 2018;61:10017–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
