Targeted literature review of current treatments and unmet need in moderate rheumatoid arthritis in the United Kingdom
- PMID: 34080612
- PMCID: PMC8566217
- DOI: 10.1093/rheumatology/keab464
Targeted literature review of current treatments and unmet need in moderate rheumatoid arthritis in the United Kingdom
Abstract
Objectives: The burden and treatment landscape of RA is poorly understood. This research aimed to identify evidence on quality of life, caregiver burden, economic burden, treatment patterns and clinical outcomes for patients with moderate RA in the United Kingdom.
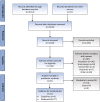
Methods: A systematic literature review was performed across multiple databases and screened against pre-defined inclusion criteria.
Results: A total of 2610 records were screened; seven studies presenting evidence for moderate RA were included. These patients were found to incur substantial burden, with moderate to severe levels of disability. Compared with patients in remission, moderate RA patients reported higher levels of disability and decreased EQ-5D utility scores. The majority of patients did not feel that their current therapy adequately controlled their disease or provided sufficient symptom relief. In the United Kingdom, the National Institute for Health and Care Excellence (NICE) have not approved advanced therapies (such as biological disease-modifying anti-rheumatic drugs) for patients with moderate disease, which restricts access for these patients.
Conclusion: The evidence available on the burden of moderate RA is limited. Despite current treatments, moderate RA still has a substantial negative impact, given that a DAS28 disease activity score defined as being in the moderate range does not qualify them for access to advanced therapies in the United Kingdom. For these patients, there is a particular need for further studies that investigate their burden and the impact of treating them earlier. Such information would help guide future treatment decisions and ensure the most effective use of resources to gain the best outcomes for patients with moderate RA.
Keywords: clinical burden; economic burden; moderate; rheumatoid arthritis; treatment; unmet need.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
References
-
- Amaya-Amaya J, Rojas-Villarraga A, Mantilla RD. et al. Rheumatoid arthritis. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, eds . Autoimmunity: from bench to bedside. Chapter 24. Bogota (Colombia: ): El Rosario University Press, 2013. - PubMed
-
- Dugowson CE. Rheumatoid arthritis. In: Goldman MB, Hatch MC, eds. Women and health. Academic Press, 2000: 674–85.
-
- Martin CR, Preedy VR.. Scientific basis of healthcare: arthritis. Boca Raton, Florida, US: Taylor & Francis, 2012.
-
- National Institute for Health and Clinical Excellence. Rheumatoid arthritis in adults: management. NICE guideline 100. 2018. https://www.nice.org.uk/guidance/ng100/resources/rheumatoid-arthritis-in....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

