Ca2+-dependent protein acyltransferase DHHC21 controls activation of CD4+ T cells
- PMID: 34080635
- PMCID: PMC8214660
- DOI: 10.1242/jcs.258186
Ca2+-dependent protein acyltransferase DHHC21 controls activation of CD4+ T cells
Abstract
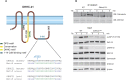
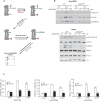
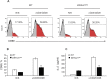
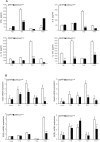
Despite the recognized significance of reversible protein lipidation (S-acylation) for T cell receptor signal transduction, the enzymatic control of this post-translational modification in T cells remains poorly understood. Here, we demonstrate that DHHC21 (also known as ZDHHC21), a member of the DHHC family of mammalian protein acyltransferases, mediates T cell receptor-induced S-acylation of proximal T cell signaling proteins. Using Zdhhc21dep mice, which express a functionally deficient version of DHHC21, we show that DHHC21 is a Ca2+/calmodulin-dependent enzyme critical for activation of naïve CD4+ T cells in response to T cell receptor stimulation. We find that disruption of the Ca2+/calmodulin-binding domain of DHHC21 does not affect thymic T cell development but prevents differentiation of peripheral CD4+ T cells into Th1, Th2 and Th17 effector T helper lineages. Our findings identify DHHC21 as an essential component of the T cell receptor signaling machinery and define a new role for protein acyltransferases in regulation of T cell-mediated immunity.
Keywords: Calmodulin; Palmitoylation; Protein acyltransferase; S-acylation; T cell receptor.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
-
- Beard, R. S., Yang, X., Meegan, J. E., Overstreet, J. W., Yang, C. G. Y., Elliott, J. A., Reynolds, J. J., Cha, B. J., Pivetti, C. D., Mitchell, D. A.et al. (2016). Palmitoyl acyltransferase DHHC21 mediates endothelial dysfunction in systemic inflammatory response syndrome. Nat. Commun. 7, 12823. 10.1038/ncomms12823 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

