Low‑calorie sweetener D‑psicose promotes hydrogen peroxide‑mediated apoptosis in C2C12 myogenic cells favoring skeletal muscle cell injury
- PMID: 34080650
- PMCID: PMC8170266
- DOI: 10.3892/mmr.2021.12175
Low‑calorie sweetener D‑psicose promotes hydrogen peroxide‑mediated apoptosis in C2C12 myogenic cells favoring skeletal muscle cell injury
Abstract
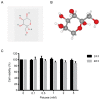
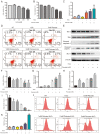
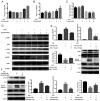
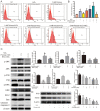
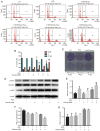
Diet and exercise are the most effective approaches used to induce weight loss. D‑psicose is a low‑calorie sweetener that has been shown to reduce weight in obese individuals. However, the effect of D‑psicose on muscle cells under oxidative stress, which is produced during exercise, requires further investigation. The present study aimed to determine the effects of D‑psicose on C2C12 myogenic cells in vitro. Hydrogen peroxide (H2O2) was used to stimulate the generation of intracellular reactive oxygen species (ROS) in muscle cells to mimic exercise conditions. Cell viability was analyzed using a MTT assay and flow cytometry was used to analyze the levels of apoptosis, mitochondrial membrane potential (MMP), the generation of ROS and the cell cycle distribution following treatment. Furthermore, protein expression levels were analyzed using western blotting and cell proliferation was determined using a colony formation assay. The results of the present study revealed that D‑psicose alone exerted no toxicity on C2C12 mouse myogenic cells. However, in the presence of low‑dose (100 µM) H2O2‑induced ROS, D‑psicose induced C2C12 cell injury and significantly decreased C2C12 cell viability in a dose‑dependent manner. In addition, the levels of apoptosis and the generation of ROS increased, while the MMP decreased. MAPK family molecules were also activated in a dose‑dependent manner following treatment. Notably, the combined treatment induced G2/M phase arrest and reduced the proliferation of C2C12 cells. In conclusion, the findings of the present study suggested that D‑psicose may induce toxic effects on muscle cells in a simulated exercise situation by increasing ROS levels, activating the MAPK signaling pathway and disrupting the MMP.
Keywords: D‑psicose; MAPK signaling pathway; apoptosis; muscle cell; reactive oxygen species.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Sui L, Dong Y, Watanabe Y, Yamaguchi F, Hatano N, Tsukamoto I, Izumori K, Tokuda M. The inhibitory effect and possible mechanisms of D-allose on cancer cell proliferation. Int J Oncol. 2005;27:907–912. - PubMed

