Diethynyl Phosphinates for Cysteine-Selective Protein Labeling and Disulfide Rebridging
- PMID: 34080747
- PMCID: PMC8362001
- DOI: 10.1002/anie.202100683
Diethynyl Phosphinates for Cysteine-Selective Protein Labeling and Disulfide Rebridging
Abstract
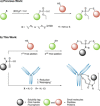
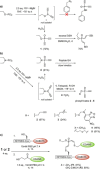
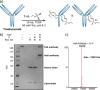
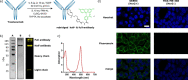
Diethynyl phosphinates were developed as bisfunctional electrophiles for the site-selective modification of peptides, proteins and antibodies. One of their electron-deficient triple bonds reacts selectively with a thiol and positions an electrophilic moiety for a subsequent intra- or intermolecular reaction with another thiol. The obtained conjugates were found to be stable in human plasma and in the presence of small thiols. We further demonstrate that this method is suitable for the generation of functional protein conjugates for intracellular delivery. Finally, this reagent class was used to generate functional homogeneously rebridged antibodies that remain specific for their target. Their modular synthesis, thiol selectivity and conjugate stability make diethynyl phosphinates ideal candidates for protein conjugation for biological and pharmaceutical applications.
Keywords: antibody rebridging; bioconjugation; cysteine-selective modification; phosphorous-based electrophiles; protein double-modification.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The chemistry described in this manuscript is part of a patent application (Appl. Number: EP21170097.6).
Figures

References
-
- Hoyt E. A., Cal P. M. S. D., Oliveira B. L., Bernardes G. J. L., Nat. Rev. Chem. 2019, 3, 147–171.
-
- Schumacher D., Hackenberger C. P. R., Curr. Opin. Chem. Biol. 2014, 22, 62–69. - PubMed
-
- Gunnoo S. B., Madder A., ChemBioChem 2016, 17, 529–553. - PubMed
-
- Ochtrop P., Hackenberger C. P. R., Curr. Opin. Chem. Biol. 2020, 58, 28–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

