Effects of sedative psychotropic drugs combined with oxycodone on respiratory depression in the rat
- PMID: 34080766
- PMCID: PMC8604244
- DOI: 10.1111/cts.13080
Effects of sedative psychotropic drugs combined with oxycodone on respiratory depression in the rat
Abstract
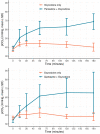
Following a decision to require label warnings for concurrent use of opioids and benzodiazepines and increased risk of respiratory depression and death, the US Food and Drug Administratioin (FDA) recognized that other sedative psychotropic drugs may be substituted for benzodiazepines and be used concurrently with opioids. In some cases, data on the ability of these alternatives to depress respiration alone or in conjunction with an opioid are lacking. A nonclinical in vivo model was developed that could detect worsening respiratory depression when a benzodiazepine (diazepam) was used in combination with an opioid (oxycodone) compared to the opioid alone based on an increased arterial partial pressure of carbon dioxide (pCO2 ). The current study used that model to assess the impact on respiration of non-benzodiazepine sedative psychotropic drugs representative of different drug classes (clozapine, quetiapine, risperidone, zolpidem, trazodone, carisoprodol, cyclobenzaprine, mirtazapine, topiramate, paroxetine, duloxetine, ramelteon, and suvorexant) administered alone and with oxycodone. At clinically relevant exposures, paroxetine, trazodone, and quetiapine given with oxycodone significantly increased pCO2 above the oxycodone effect. Analyses indicated that most pCO2 interaction effects were due to pharmacokinetic interactions resulting in increased oxycodone exposure. Increased pCO2 recorded with oxycodone-paroxetine co-administration exceeded expected effects from only drug exposure suggesting another mechanism for the increased pharmacodynamic response. This study identified drug-drug interaction effects depressing respiration in an animal model when quetiapine or paroxetine were co-administered with oxycodone. Clinical pharmacodynamic drug interaction studies are being conducted with these drugs to assess translatability of these findings.
Published 2021. This article is a U.S. Government work and is in the public domain in the USA. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures

References
-
- SAMSA: key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. 2020. https://www.samhsa.gov/data/sites/default/files/cbhsq‐reports/NSDUHNatio.... Accessed March 17, 2020.
-
- Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief, no 356. Hyattsville, MD: National Center for Health Statistics; 2020.
-
- NIDA: overdose death rates (attached supplemental document: drug overdoses data document), revised March 2020. National Institute of Drug Abuse. 2020. https://www.drugabuse.gov/related‐topics/trends‐statistics/overdose‐deat.... Accessed March 17, 2020.
-
- Baltimore Commissioner of Health , Citizens petition. 2016. http://health.baltimorecity.gov/sites/default/files/Final%20Draft%20FDA%.... Accessed March 12, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

