Effect of Bamlanivimab vs Placebo on Incidence of COVID-19 Among Residents and Staff of Skilled Nursing and Assisted Living Facilities: A Randomized Clinical Trial
- PMID: 34081073
- PMCID: PMC8176388
- DOI: 10.1001/jama.2021.8828
Effect of Bamlanivimab vs Placebo on Incidence of COVID-19 Among Residents and Staff of Skilled Nursing and Assisted Living Facilities: A Randomized Clinical Trial
Abstract
Importance: Preventive interventions are needed to protect residents and staff of skilled nursing and assisted living facilities from COVID-19 during outbreaks in their facilities. Bamlanivimab, a neutralizing monoclonal antibody against SARS-CoV-2, may confer rapid protection from SARS-CoV-2 infection and COVID-19.
Objective: To determine the effect of bamlanivimab on the incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities.
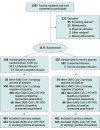
Design, setting, and participants: Randomized, double-blind, single-dose, phase 3 trial that enrolled residents and staff of 74 skilled nursing and assisted living facilities in the United States with at least 1 confirmed SARS-CoV-2 index case. A total of 1175 participants enrolled in the study from August 2 to November 20, 2020. Database lock was triggered on January 13, 2021, when all participants reached study day 57.
Interventions: Participants were randomized to receive a single intravenous infusion of bamlanivimab, 4200 mg (n = 588), or placebo (n = 587).
Main outcomes and measures: The primary outcome was incidence of COVID-19, defined as the detection of SARS-CoV-2 by reverse transcriptase-polymerase chain reaction and mild or worse disease severity within 21 days of detection, within 8 weeks of randomization. Key secondary outcomes included incidence of moderate or worse COVID-19 severity and incidence of SARS-CoV-2 infection.
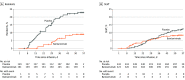
Results: The prevention population comprised a total of 966 participants (666 staff and 300 residents) who were negative at baseline for SARS-CoV-2 infection and serology (mean age, 53.0 [range, 18-104] years; 722 [74.7%] women). Bamlanivimab significantly reduced the incidence of COVID-19 in the prevention population compared with placebo (8.5% vs 15.2%; odds ratio, 0.43 [95% CI, 0.28-0.68]; P < .001; absolute risk difference, -6.6 [95% CI, -10.7 to -2.6] percentage points). Five deaths attributed to COVID-19 were reported by day 57; all occurred in the placebo group. Among 1175 participants who received study product (safety population), the rate of participants with adverse events was 20.1% in the bamlanivimab group and 18.9% in the placebo group. The most common adverse events were urinary tract infection (reported by 12 participants [2%] who received bamlanivimab and 14 [2.4%] who received placebo) and hypertension (reported by 7 participants [1.2%] who received bamlanivimab and 10 [1.7%] who received placebo).
Conclusions and relevance: Among residents and staff in skilled nursing and assisted living facilities, treatment during August-November 2020 with bamlanivimab monotherapy reduced the incidence of COVID-19 infection. Further research is needed to assess preventive efficacy with current patterns of viral strains with combination monoclonal antibody therapy.
Trial registration: ClinicalTrials.gov Identifier: NCT04497987.
Conflict of interest statement
Figures

Comment in
-
Bamlanivimab for Prevention of COVID-19.JAMA. 2021 Jul 6;326(1):31-32. doi: 10.1001/jama.2021.7515. JAMA. 2021. PMID: 34081075 No abstract available.
References
-
- McMichael TM, Currie DW, Clark S, et al. ; Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team . Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. doi: 10.1056/NEJMoa2005412 - DOI - PMC - PubMed
-
- Kaiser Family Foundation . State COVID-19 data and policy actions. Published May 18, 2021. Accessed February 7, 2021. https://www.kff.org/coronavirus-covid-19/issue-brief/state-covid-19-data...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 AI047996/AI/NIAID NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- U01 AI068635/AI/NIAID NIH HHS/United States
- U01 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- UM1 AI148574/AI/NIAID NIH HHS/United States
- UM1 AI068617/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

