Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis
- PMID: 34081398
- PMCID: PMC8542957
- DOI: 10.1111/irv.12871
Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis
Abstract
Background: Standard-dose seasonal influenza vaccines often produce modest immunogenic responses in adults ≥65 years old. MF59 is intended to elicit a greater magnitude and increased breadth of immune response.
Objective: To determine the effectiveness of seasonal MF59-adjuvanted trivalent/quadrivalent influenza vaccine (aTIV/aQIV) relative to no vaccination or vaccination with standard or high-dose egg-based influenza vaccines among people ≥65 years old.
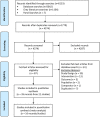
Methods: Cochrane methodological standards and PRISMA-P guidelines were followed. Real-world evidence from non-interventional studies published in peer-reviewed journals and gray literature from 1997 through to July 15, 2020, including cluster-randomized trials, were eligible. Two reviewers independently extracted data; risk of bias was assessed using the ROBINS-I tool.
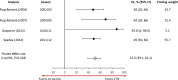
Results: Twenty-one studies conducted during the 2006/07-2019/20 influenza seasons were included in the qualitative review; 16 in the meta-analyses. Meta-analysis of test-negative studies found that aTIV reduced medical encounters due to lab-confirmed influenza with pooled estimates of 40.7% (95% CI: 21.9, 54.9; I2 = 0%) for non-emergency outpatient visits and 58.5% (40.7, 70.9; I2 = 52.9%) for hospitalized patients. The pooled estimate of VE from case-control studies was 51.3% (39.1, 61.1; I2 = 0%) against influenza- or pneumonia-related hospitalization. The pooled estimates for the relative VE of aTIV for the prevention of influenza-related medical encounters were 13.9% (4.2, 23.5; I2 = 95.9%) compared with TIV, 13.7% (3.1, 24.2; I2 = 98.8%) compared with QIV, and 2.8% (-2.9, 8.5; I2 = 94.5%) compared with HD-TIV.
Conclusions: Among adults ≥65 years, aTIV demonstrated significant absolute VE, improved relative VE compared to non-adjuvanted standard-dose TIV/QIV, and comparable relative VE to high-dose TIV.
Keywords: MF59-adjuvanted influenza vaccine; older adults; real-world evidence; systematic literature review; vaccine effectiveness.
© 2021 The Authors. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
BLC and RS were employed by Sinai Health which received funding from Seqirus for this review. MDMH and IM are employed by Seqirus.
Figures


Comment in
-
Letter to editor regarding the systematic review and meta-analysis by Coleman et al. on the effectiveness of MF59-adjuvanted seasonal influenza vaccine in older adults.Influenza Other Respir Viruses. 2021 Nov;15(6):826-827. doi: 10.1111/irv.12891. Epub 2021 Aug 2. Influenza Other Respir Viruses. 2021. PMID: 34342136 Free PMC article. No abstract available.
-
Reply to Letter to the Editor by Yin et al.Influenza Other Respir Viruses. 2021 Nov;15(6):828-829. doi: 10.1111/irv.12895. Epub 2021 Aug 10. Influenza Other Respir Viruses. 2021. PMID: 34378326 Free PMC article. No abstract available.
References
-
- World Health Organization . Global influenza strategy 2019–2030. 2019.
-
- Centres for Disease Control and Prevention . Estimates of deaths associated with seasonal influenza – United States, 1976–2007. MMWR Morb Mortal Wkly Rep. 2010;59(33):1057‐1062. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

