Myosin dilated cardiomyopathy mutation S532P disrupts actomyosin interactions, leading to altered muscle kinetics, reduced locomotion, and cardiac dilation in Drosophila
- PMID: 34081531
- PMCID: PMC8684735
- DOI: 10.1091/mbc.E21-02-0088
Myosin dilated cardiomyopathy mutation S532P disrupts actomyosin interactions, leading to altered muscle kinetics, reduced locomotion, and cardiac dilation in Drosophila
Abstract
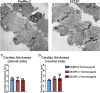
Dilated cardiomyopathy (DCM), a life-threatening disease characterized by pathological heart enlargement, can be caused by myosin mutations that reduce contractile function. To better define the mechanistic basis of this disease, we employed the powerful genetic and integrative approaches available in Drosophila melanogaster. To this end, we generated and analyzed the first fly model of human myosin-induced DCM. The model reproduces the S532P human β-cardiac myosin heavy chain DCM mutation, which is located within an actin-binding region of the motor domain. In concordance with the mutation's location at the actomyosin interface, steady-state ATPase and muscle mechanics experiments revealed that the S532P mutation reduces the rates of actin-dependent ATPase activity and actin binding and increases the rate of actin detachment. The depressed function of this myosin form reduces the number of cross-bridges during active wing beating, the power output of indirect flight muscles, and flight ability. Further, S532P mutant hearts exhibit cardiac dilation that is mutant gene dose-dependent. Our study shows that Drosophila can faithfully model various aspects of human DCM phenotypes and suggests that impaired actomyosin interactions in S532P myosin induce contractile deficits that trigger the disease.
Figures





Similar articles
-
The R249Q hypertrophic cardiomyopathy myosin mutation decreases contractility in Drosophila by impeding force production.J Physiol. 2019 May;597(9):2403-2420. doi: 10.1113/JP277333. Epub 2019 Apr 4. J Physiol. 2019. PMID: 30950055 Free PMC article.
-
Drosophila myosin mutants model the disparate severity of type 1 and type 2B distal arthrogryposis and indicate an enhanced actin affinity mechanism.Skelet Muscle. 2020 Aug 15;10(1):24. doi: 10.1186/s13395-020-00241-6. Skelet Muscle. 2020. PMID: 32799913 Free PMC article.
-
Dilated cardiomyopathy myosin mutants have reduced force-generating capacity.J Biol Chem. 2018 Jun 8;293(23):9017-9029. doi: 10.1074/jbc.RA118.001938. Epub 2018 Apr 17. J Biol Chem. 2018. PMID: 29666183 Free PMC article.
-
Genetic mutations and mechanisms in dilated cardiomyopathy.J Clin Invest. 2013 Jan;123(1):19-26. doi: 10.1172/JCI62862. Epub 2013 Jan 2. J Clin Invest. 2013. PMID: 23281406 Free PMC article. Review.
-
Effects of hypertrophic and dilated cardiomyopathy mutations on power output by human β-cardiac myosin.J Exp Biol. 2016 Jan;219(Pt 2):161-7. doi: 10.1242/jeb.125930. J Exp Biol. 2016. PMID: 26792326 Free PMC article. Review.
Cited by
-
The self-oscillation paradox in the flight motor of Drosophila melanogaster.J R Soc Interface. 2023 Nov;20(208):20230421. doi: 10.1098/rsif.2023.0421. Epub 2023 Nov 15. J R Soc Interface. 2023. PMID: 37963559 Free PMC article.
-
Drosophila Models Reveal Properties of Mutant Lamins That Give Rise to Distinct Diseases.Cells. 2023 Apr 12;12(8):1142. doi: 10.3390/cells12081142. Cells. 2023. PMID: 37190051 Free PMC article.
-
The R369 Myosin Residue within Loop 4 Is Critical for Actin Binding and Muscle Function in Drosophila.Int J Mol Sci. 2022 Feb 25;23(5):2533. doi: 10.3390/ijms23052533. Int J Mol Sci. 2022. PMID: 35269675 Free PMC article.
-
Assessing Cardiac Contractility From Single Molecules to Whole Hearts.JACC Basic Transl Sci. 2023 Oct 11;9(3):414-439. doi: 10.1016/j.jacbts.2023.07.013. eCollection 2024 Mar. JACC Basic Transl Sci. 2023. PMID: 38559627 Free PMC article. Review.
-
Small Angle X-ray Diffraction as a Tool for Structural Characterization of Muscle Disease.Int J Mol Sci. 2022 Mar 11;23(6):3052. doi: 10.3390/ijms23063052. Int J Mol Sci. 2022. PMID: 35328477 Free PMC article. Review.
References
-
- Achal M, Trujillo AS, Melkani GC, Farman GP, Ocorr K, Viswanathan MC, Kaushik G, Newhard CS, Glasheen BM, Melkani A, et al. (2016). A restrictive cardiomyopathy mutation in an invariant proline at the myosin head/rod junction enhances head flexibility and function, yielding muscle defects in Drosophila. J Mol Biol 428, 2446–2461. - PMC - PubMed
-
- Bernstein SI, Mogami K, Donady JJ, Emerson CP Jr. (1983). Drosophila muscle myosin heavy chain encoded by a single gene in a cluster of muscle mutations. Nature 302, 393–397. - PubMed
-
- Bernstein SI, O’Donnell PT, Cripps RM (1993). Molecular genetic analysis of muscle development, structure, and function in Drosophila. Int Rev Cytol 143, 63–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

