Calcium signaling mediates a biphasic mechanoadaptive response of endothelial cells to cyclic mechanical stretch
- PMID: 34081532
- PMCID: PMC8684738
- DOI: 10.1091/mbc.E21-03-0106
Calcium signaling mediates a biphasic mechanoadaptive response of endothelial cells to cyclic mechanical stretch
Abstract
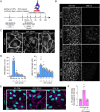
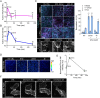
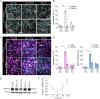
The vascular system is precisely regulated to adjust blood flow to organismal demand, thereby guaranteeing adequate perfusion under varying physiological conditions. Mechanical forces, such as cyclic circumferential stretch, are among the critical stimuli that dynamically adjust vessel distribution and diameter, but the precise mechanisms of adaptation to changing forces are unclear. We find that endothelial monolayers respond to cyclic stretch by transient remodeling of the vascular endothelial cadherin-based adherens junctions and the associated actomyosin cytoskeleton. Time-resolved proteomic profiling reveals that this remodeling is driven by calcium influx through the mechanosensitive Piezo1 channel, triggering Rho activation to increase actomyosin contraction. As the mechanical stimulus persists, calcium signaling is attenuated through transient down-regulation of Piezo1 protein. At the same time, filamins are phosphorylated to increase monolayer stiffness, allowing mechanoadaptation to restore junctional integrity despite continuing exposure to stretch. Collectively, this study identifies a biphasic response to cyclic stretch, consisting of an initial calcium-driven junctional mechanoresponse, followed by mechanoadaptation facilitated by monolayer stiffening.
Figures



Similar articles
-
A Mechanosensitive RhoA Pathway that Protects Epithelia against Acute Tensile Stress.Dev Cell. 2018 Nov 19;47(4):439-452.e6. doi: 10.1016/j.devcel.2018.09.016. Epub 2018 Oct 11. Dev Cell. 2018. PMID: 30318244
-
Mechanical tugging force regulates the size of cell-cell junctions.Proc Natl Acad Sci U S A. 2010 Jun 1;107(22):9944-9. doi: 10.1073/pnas.0914547107. Epub 2010 May 12. Proc Natl Acad Sci U S A. 2010. PMID: 20463286 Free PMC article.
-
Adherens Junction Length during Tissue Contraction Is Controlled by the Mechanosensitive Activity of Actomyosin and Junctional Recycling.Dev Cell. 2018 Nov 19;47(4):453-463.e3. doi: 10.1016/j.devcel.2018.10.025. Dev Cell. 2018. PMID: 30458138 Free PMC article.
-
Dynamic Regulation of Vascular Permeability by Vascular Endothelial Cadherin-Mediated Endothelial Cell-Cell Junctions.J Nippon Med Sch. 2017;84(4):148-159. doi: 10.1272/jnms.84.148. J Nippon Med Sch. 2017. PMID: 28978894 Review.
-
Active tension: the role of cadherin adhesion and signaling in generating junctional contractility.Curr Top Dev Biol. 2015;112:65-102. doi: 10.1016/bs.ctdb.2014.11.016. Epub 2015 Feb 12. Curr Top Dev Biol. 2015. PMID: 25733138 Review.
Cited by
-
Understanding the role of vascular stretch on modulation of VWF and ANGPT-2 in continuous flow left ventricular assist device (CF-VAD) patients.Lab Chip. 2025 May 28;25(11):2722-2731. doi: 10.1039/d4lc01065e. Lab Chip. 2025. PMID: 40314578 Free PMC article.
-
A Novel Single-Color FRET Sensor for Rho-Kinase Reveals Calcium-Dependent Activation of RhoA and ROCK.Sensors (Basel). 2024 Oct 26;24(21):6869. doi: 10.3390/s24216869. Sensors (Basel). 2024. PMID: 39517770 Free PMC article.
-
StretchView - A Multi-Axial Cell-Stretching Device for Long-Term Automated Videomicroscopy of Living Cells.Adv Sci (Weinh). 2025 Mar;12(9):e2408853. doi: 10.1002/advs.202408853. Epub 2025 Jan 10. Adv Sci (Weinh). 2025. PMID: 39792792 Free PMC article.
-
Vascular mechanotransduction.Physiol Rev. 2023 Apr 1;103(2):1247-1421. doi: 10.1152/physrev.00053.2021. Epub 2023 Jan 5. Physiol Rev. 2023. PMID: 36603156 Free PMC article. Review.
-
Calcium Unified: Understanding How Calcium's Atomic Properties Impact Human Health.Cells. 2025 Jul 11;14(14):1066. doi: 10.3390/cells14141066. Cells. 2025. PMID: 40710319 Free PMC article. Review.
References
-
- Abriel H, Staub O (2005). Ubiquitylation of ion channels. Physiology (Bethesda) 20, 398–407. - PubMed
-
- Acharya BR, Nestor-Bergmann A, Liang X, Gupta S, Duszyc K, Gauquelin E, Gomez GA, Budnar S, Marcq P, Jensen OE, et al. (2018). A mechanosensitive RhoA pathway that protects epithelia against acute tensile stress. Dev Cell 47, 439–452 e436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

