Stochastic contraction of myosin minifilaments drives evolution of microridge protrusion patterns in epithelial cells
- PMID: 34081537
- PMCID: PMC8351741
- DOI: 10.1091/mbc.E21-05-0258
Stochastic contraction of myosin minifilaments drives evolution of microridge protrusion patterns in epithelial cells
Abstract
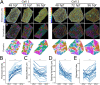
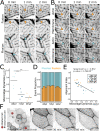
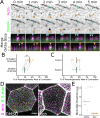
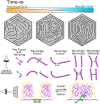
Actin-based protrusions vary in morphology, stability, and arrangement on cell surfaces. Microridges are laterally elongated protrusions on mucosal epithelial cells, where they form evenly spaced, mazelike patterns that dynamically remodel by fission and fusion. To characterize how microridges form their highly ordered, subcellular patterns and investigate the mechanisms driving fission and fusion, we imaged microridges in the maturing skin of zebrafish larvae. After their initial development, microridge spacing and alignment became increasingly well ordered. Imaging F-actin and non-muscle myosin II (NMII) revealed that microridge fission and fusion were associated with local NMII activity in the apical cortex. Inhibiting NMII blocked fission and fusion rearrangements, reduced microridge density, and altered microridge spacing. High-resolution imaging allowed us to image individual NMII minifilaments in the apical cortex of cells in live animals, revealing that minifilaments are tethered to protrusions and often connect adjacent microridges. NMII minifilaments connecting the ends of two microridges fused them together, whereas minifilaments oriented perpendicular to microridges severed them or pulled them closer together. These findings demonstrate that as cells mature, cortical NMII activity orchestrates a remodeling process that creates an increasingly orderly microridge arrangement.
Figures





Similar articles
-
Keratins and the plakin family cytolinker proteins control the length of epithelial microridge protrusions.Elife. 2020 Sep 7;9:e58149. doi: 10.7554/eLife.58149. Elife. 2020. PMID: 32894222 Free PMC article.
-
Cortical contraction drives the 3D patterning of epithelial cell surfaces.J Cell Biol. 2020 Mar 2;219(3):e201904144. doi: 10.1083/jcb.201904144. J Cell Biol. 2020. PMID: 32003768 Free PMC article.
-
How to wrinkle a cell: Emerging mechanisms of microridge morphogenesis.Curr Opin Cell Biol. 2022 Jun;76:102088. doi: 10.1016/j.ceb.2022.102088. Epub 2022 May 12. Curr Opin Cell Biol. 2022. PMID: 35569260 Review.
-
In vivo imaging and characterization of actin microridges.PLoS One. 2015 Jan 28;10(1):e0115639. doi: 10.1371/journal.pone.0115639. eCollection 2015. PLoS One. 2015. PMID: 25629723 Free PMC article.
-
Actin Microridges.Anat Rec (Hoboken). 2018 Dec;301(12):2037-2050. doi: 10.1002/ar.23965. Epub 2018 Nov 9. Anat Rec (Hoboken). 2018. PMID: 30414250 Review.
Cited by
-
Microridge-like structures anchor motile cilia.Nat Commun. 2022 Apr 19;13(1):2056. doi: 10.1038/s41467-022-29741-3. Nat Commun. 2022. PMID: 35440631 Free PMC article.
-
A deep learning framework for quantitative analysis of actin microridges.NPJ Syst Biol Appl. 2023 Jun 2;9(1):21. doi: 10.1038/s41540-023-00276-7. NPJ Syst Biol Appl. 2023. PMID: 37268613 Free PMC article.
-
Patterning of the cell cortex by Rho GTPases.Nat Rev Mol Cell Biol. 2024 Apr;25(4):290-308. doi: 10.1038/s41580-023-00682-z. Epub 2024 Jan 3. Nat Rev Mol Cell Biol. 2024. PMID: 38172611 Review.
-
A transient apical extracellular matrix relays cytoskeletal patterns to shape permanent acellular ridges on the surface of adult C. elegans.PLoS Genet. 2022 Aug 12;18(8):e1010348. doi: 10.1371/journal.pgen.1010348. eCollection 2022 Aug. PLoS Genet. 2022. PMID: 35960773 Free PMC article.
-
Spontaneous Calcium Bursts Organize the Apical Actin Cytoskeleton of Multiciliated Cells.Int J Mol Sci. 2025 Mar 11;26(6):2507. doi: 10.3390/ijms26062507. Int J Mol Sci. 2025. PMID: 40141151 Free PMC article.
References
-
- Costa M, Draper BW, Priess JR (1997). The role of actin filaments in patterning the Caenorhabditis elegans cuticle. Dev Biol 184, 373–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

